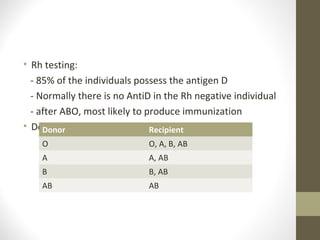
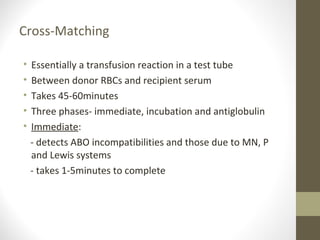
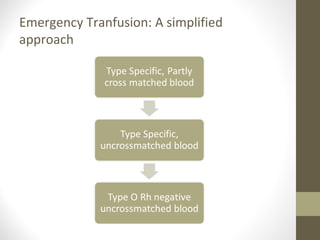
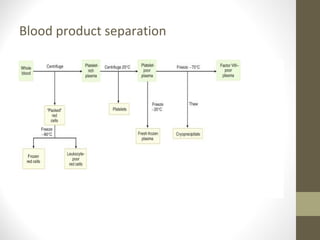
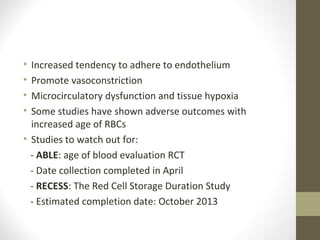
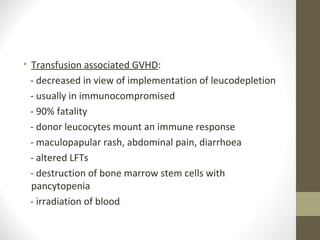
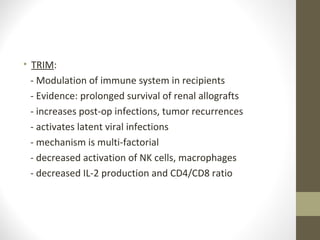
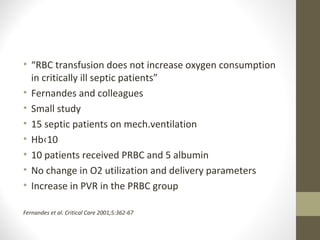
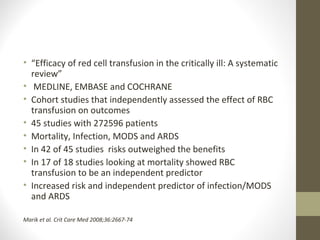
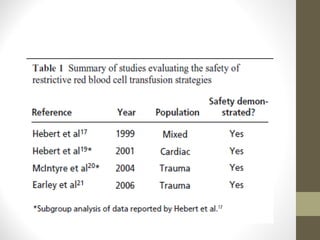
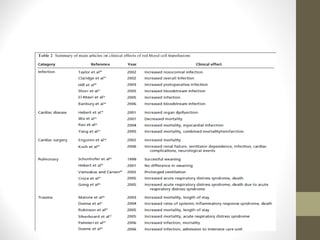
This document provides an overview of red cell transfusions in critically ill patients. It discusses the history of blood transfusions, reasons for anemia in ICU patients, physiological effects of red blood cells, technical aspects of blood compatibility testing and storage, hazards of transfusions, and evidence from studies on restrictive vs liberal transfusion thresholds. The TRICC study found no significant difference in mortality between restrictive and liberal transfusion strategies, though subgroups with lower illness severity saw benefits with restriction. Overall the document examines the risks and benefits of red cell transfusions in critical care.