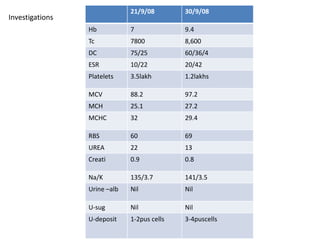
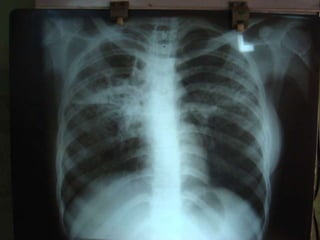
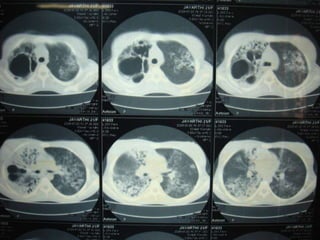
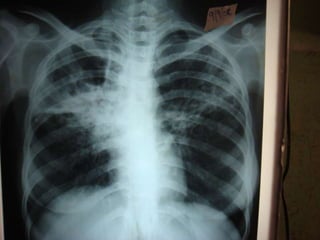
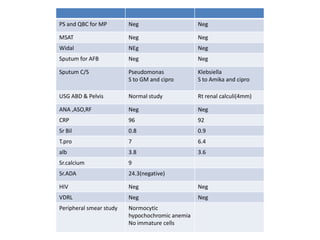
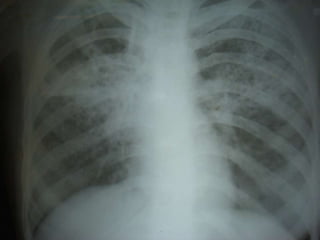
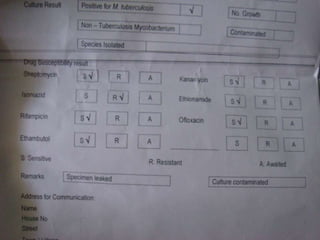
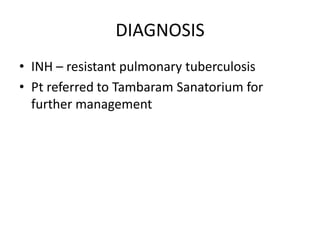
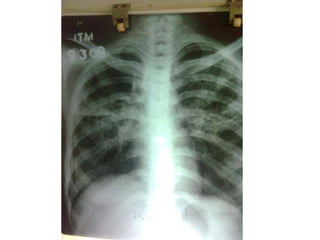
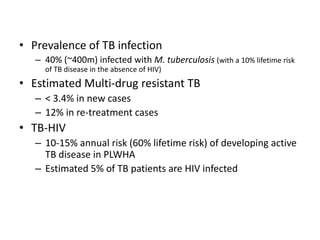
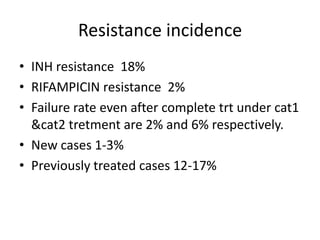
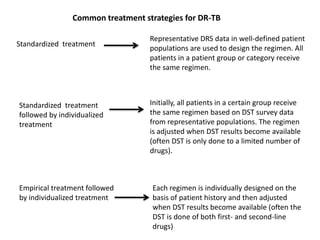
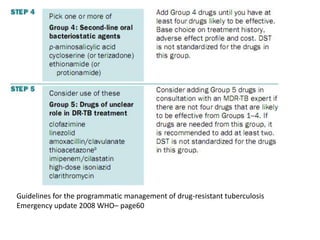
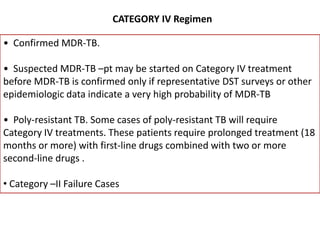
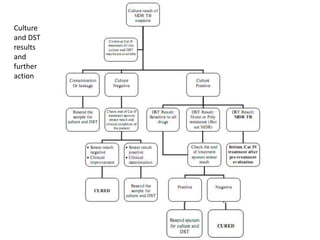
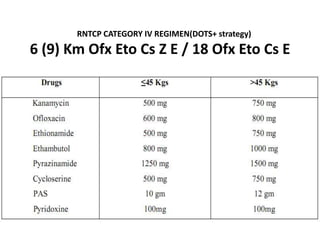
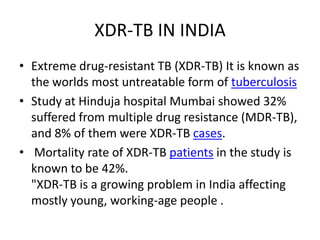
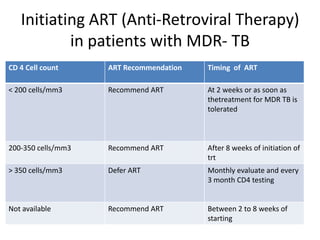
This document presents the case of a 26-year-old female patient who was admitted with a 5-month history of intermittent fever, weight loss, cough, and breathlessness. Her past medical history includes a previous episode of pulmonary tuberculosis that was treated. On examination, she had signs of respiratory involvement including reduced breath sounds and crackles on the right side. Investigations showed infiltrates on the right lung. She was started on treatment but did not improve. Further testing revealed she had inh resistant pulmonary tuberculosis. She was referred for specialized management and her diagnosis was confirmed as suspected MDR-TB.