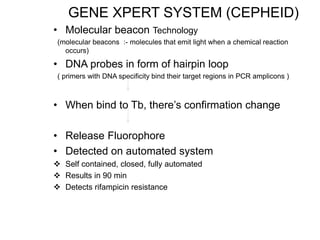
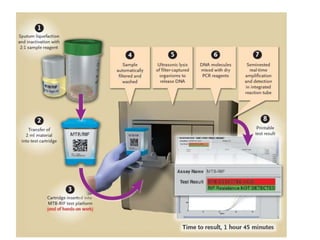
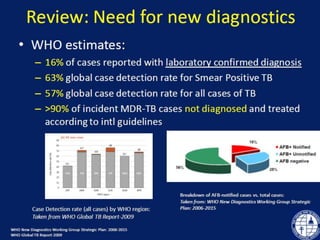
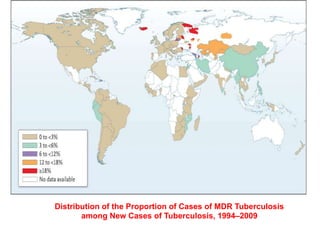
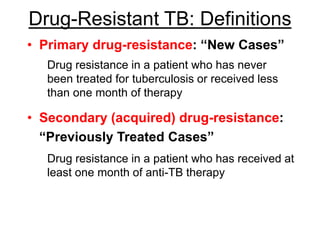
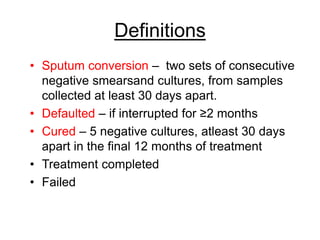
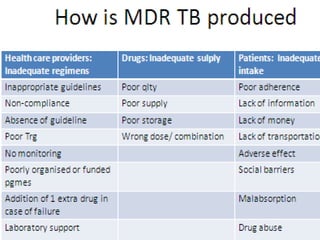
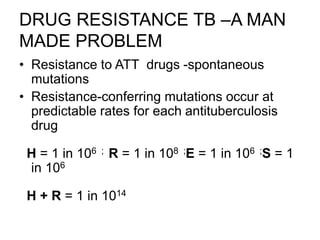
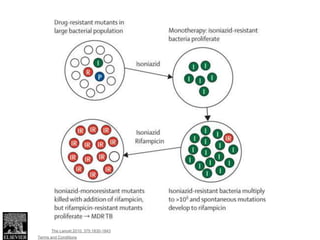
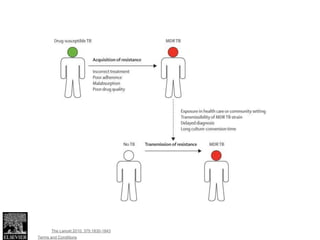
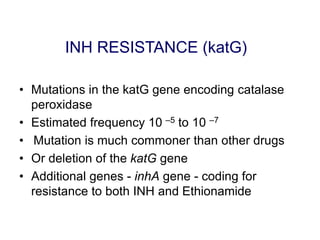
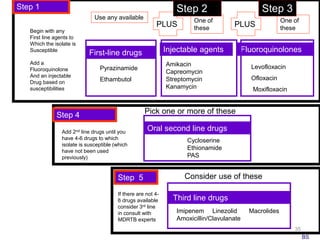
This document discusses the diagnosis and treatment of multidrug-resistant tuberculosis (MDR TB). It covers various diagnostic methods for TB including smear microscopy, culture, and rapid molecular tests like the GeneXpert system. It defines MDR TB and extensively drug-resistant TB. It notes the threat posed by increasing cases of MDR TB and discusses treatment regimens and monitoring of patients undergoing treatment for MDR TB.






















![• New patients with pulmonary TB may receive a
daily intensive phase followed by three times
weekly continuation phase [2HRZE/4(HR)3],
provided that each dose is directly observed
(Conditional/High or moderate grade of evidence)
• Three times weekly dosing throughout therapy
[2(HRZE)3/4(HR)3], provided that every dose is
directly observed therapy and the patient is
NOT living with HIV or living in an HIV-
prevalent setting
(Conditional/High or moderate grade of evidence)](https://image.slidesharecdn.com/mdrtbppt-221115114650-d95e67e5/85/MDR-TB-ppt-pptx-42-320.jpg)


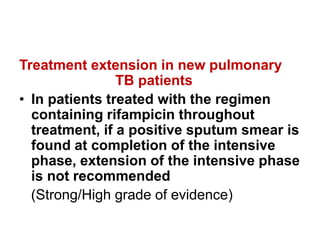








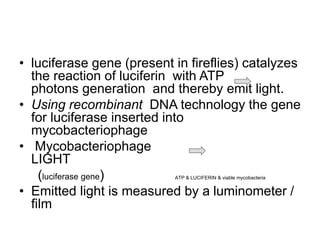

![CALORIMETRIC SYSTEMS
TK MEDIUM [SALUBRIS]
• Rapid liquid culture
• Indicator dyes change colour on growth
• SPUTUM + :- orange,
• No growth :- red
• Detects in 2 weeks](https://image.slidesharecdn.com/mdrtbppt-221115114650-d95e67e5/85/MDR-TB-ppt-pptx-66-320.jpg)