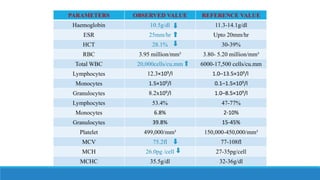
This case study presents a 3-year old female patient admitted with fever, coffee ground vomiting and abdominal pain. She had a history of upper respiratory infection and allergy to cephalosporin and amoxicillin. Endoscopy revealed a non-bleeding gastric ulcer. Laboratory tests showed signs of anemia and inflammation. She was diagnosed with NSAIDs-induced peptic ulcer disease. Her treatment plan included cefotaxime, paracetamol, esomeprazole, and pantoprazole. She was discharged after 2 days with counseling on diet, medication use, and follow up.