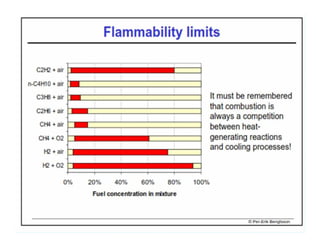
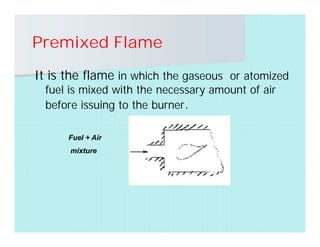
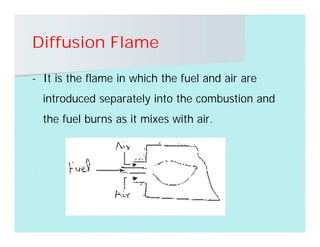
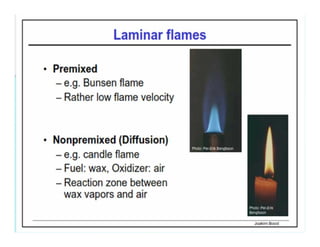
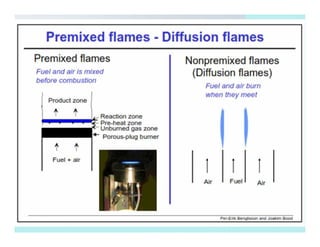
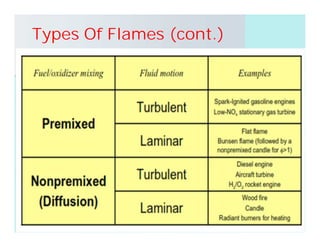
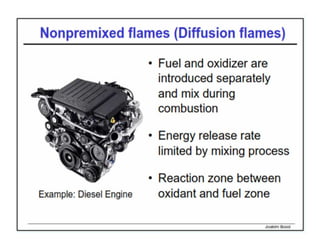
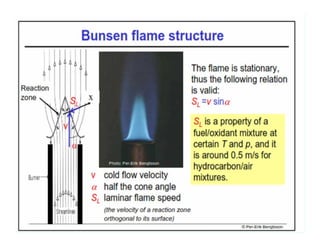
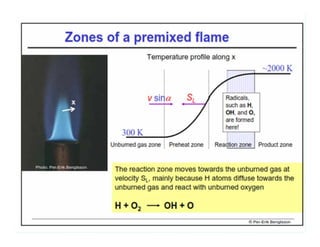
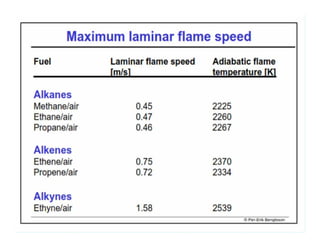
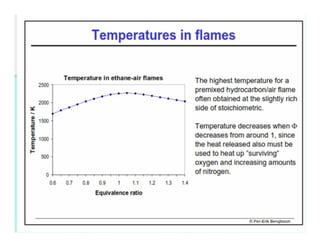
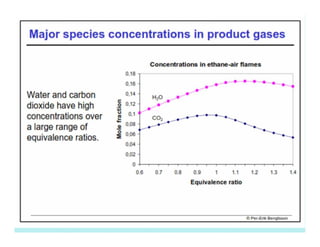
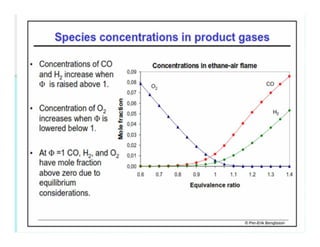
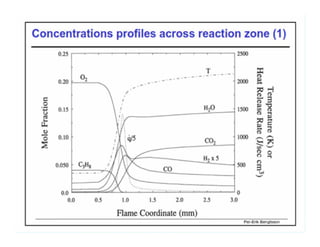
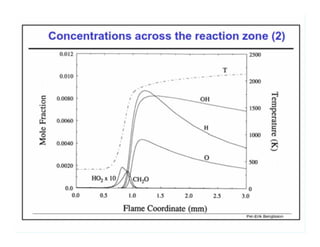
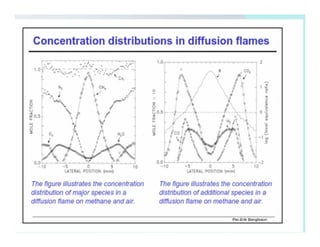
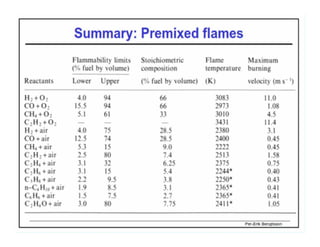
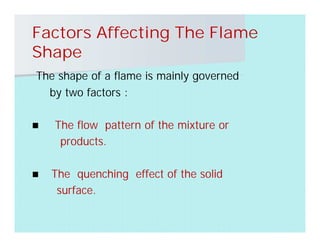
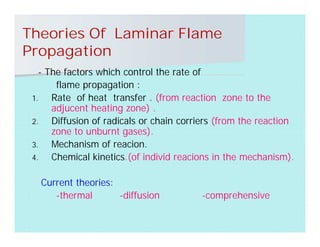
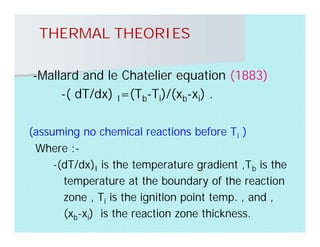
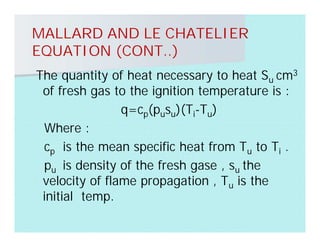
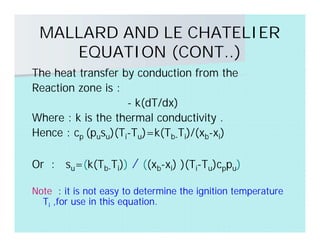
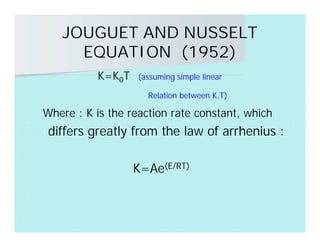
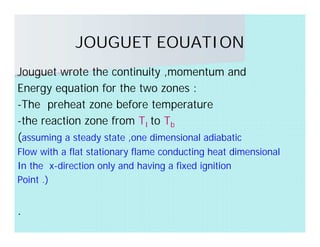
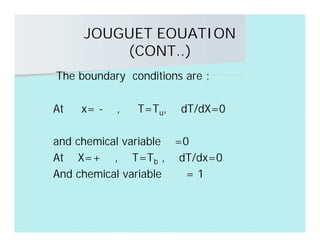
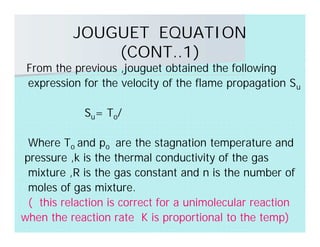
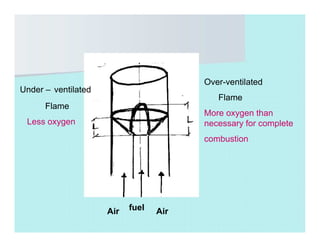
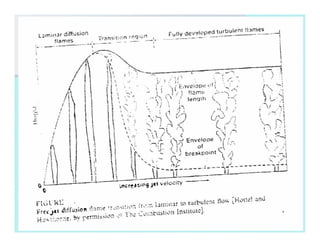
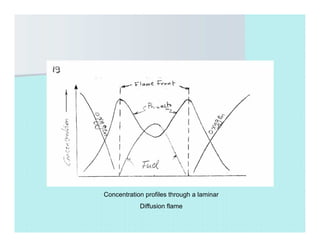
This document discusses different types of flames including premixed and diffusion flames. It defines a flame as a thermal wave where rapid exothermic chemical reactions occur and travel at subsonic velocities. Premixed flames involve fuel and air mixtures that burn, while diffusion flames involve separate introduction of fuel and air that mix and burn. The structure of laminar premixed flames is also examined, including temperature and concentration gradients across the combustion wave and factors affecting flame shape.