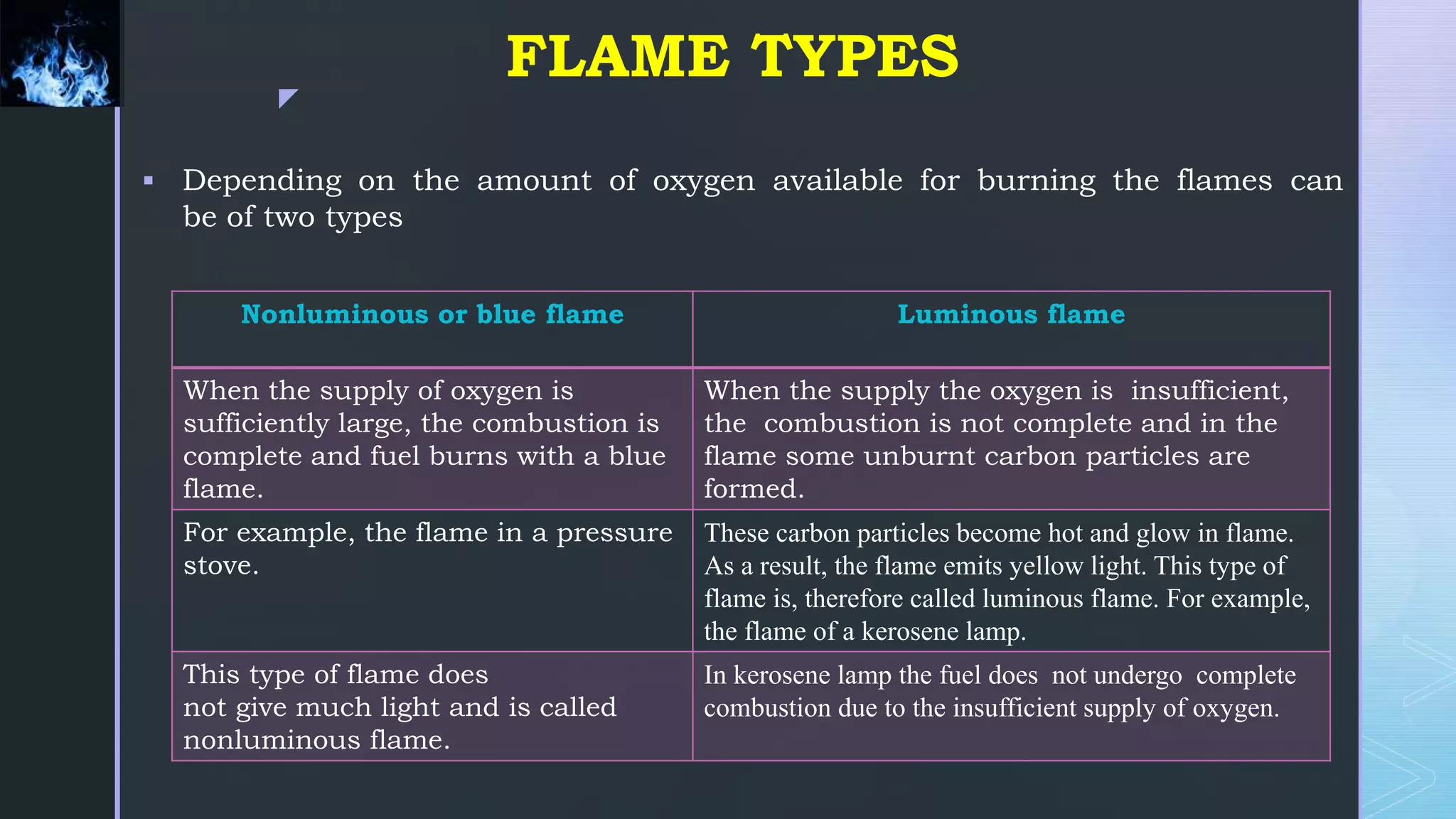
The document provides an extensive overview of flames, including their formation, types, and structures. It differentiates between luminous and non-luminous flames based on oxygen supply, as well as premixed and non-premixed flames based on how fuel and oxidizers mix before combustion. Additionally, it discusses flame characteristics such as color, temperature, and various combustion methods, including surface and submerged combustion.