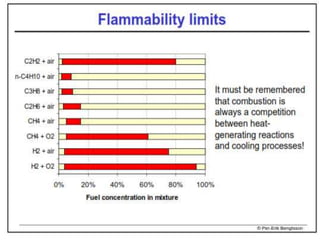
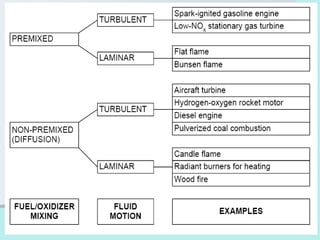
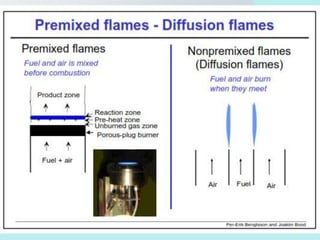
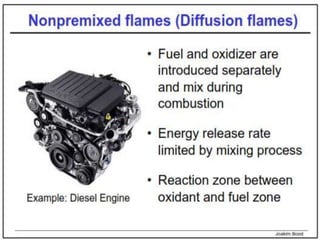
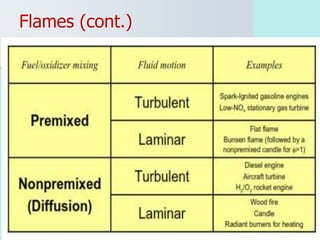
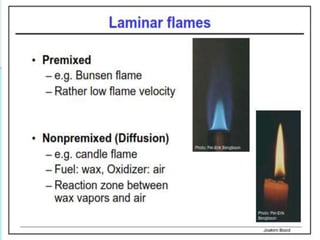
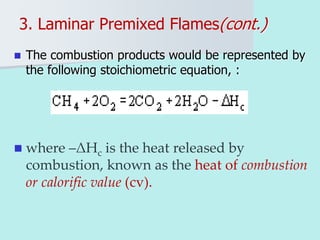
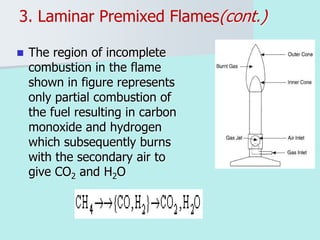
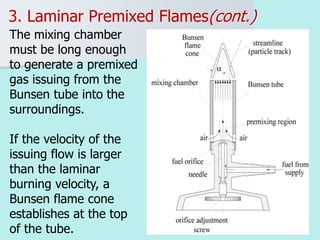
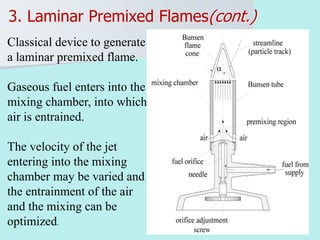
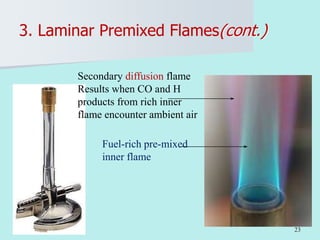
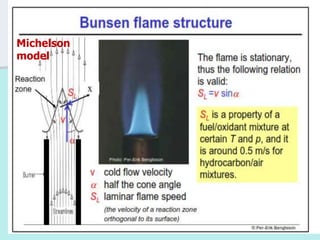
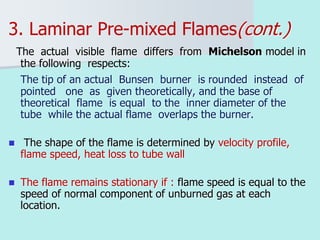
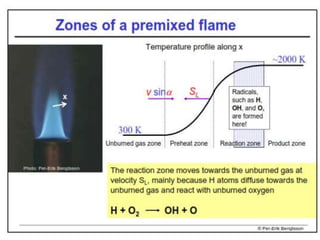
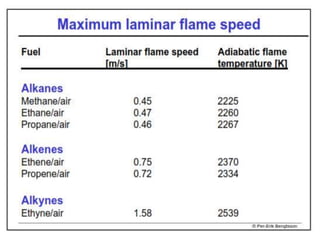
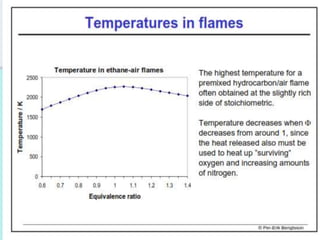
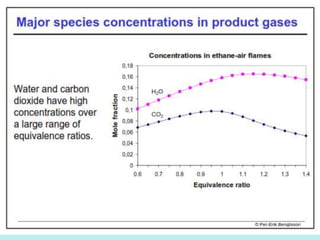
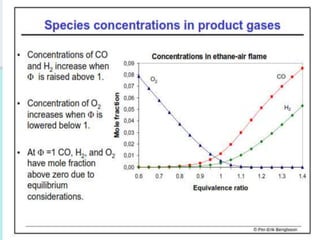
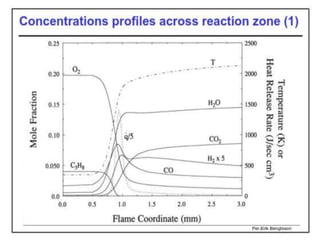
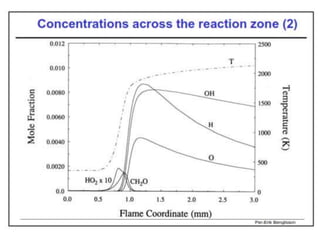
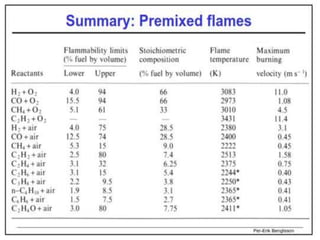
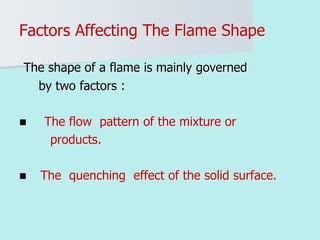
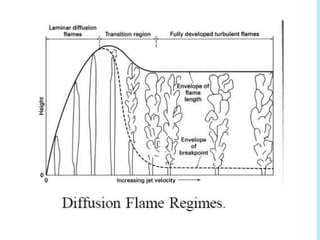
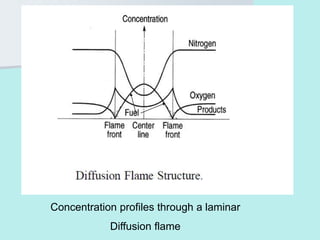
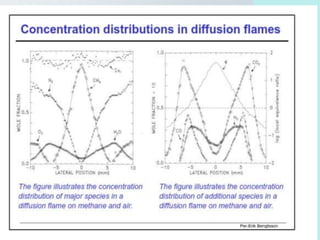
The document provides an extensive overview of flames, detailing definitions, types, and characteristics of both laminar premixed and laminar diffusion flames. It explains key concepts such as flammable mixtures, flame velocity, and propagation, while also describing how flames can be categorized based on various criteria. The text emphasizes the distinctions between different flame types and their applications, particularly in devices like bunsen burners and industrial processes.