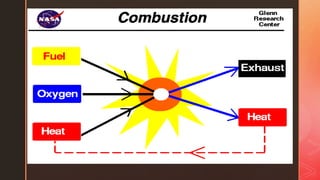
Combustion is the rapid chemical combination of a substance with oxygen, producing heat and light. Heating value or calorific value refers to the amount of heat released during combustion of a fuel. There are two types - gross calorific value includes the heat of vaporization of water, while net calorific value does not. Combustion efficiency is a measure of how well a fuel is utilized during combustion, calculated based on heat produced versus potential heat of the fuel. Factors like excess air, flue gas temperature, fuel specifications and ambient temperature can impact combustion efficiency.