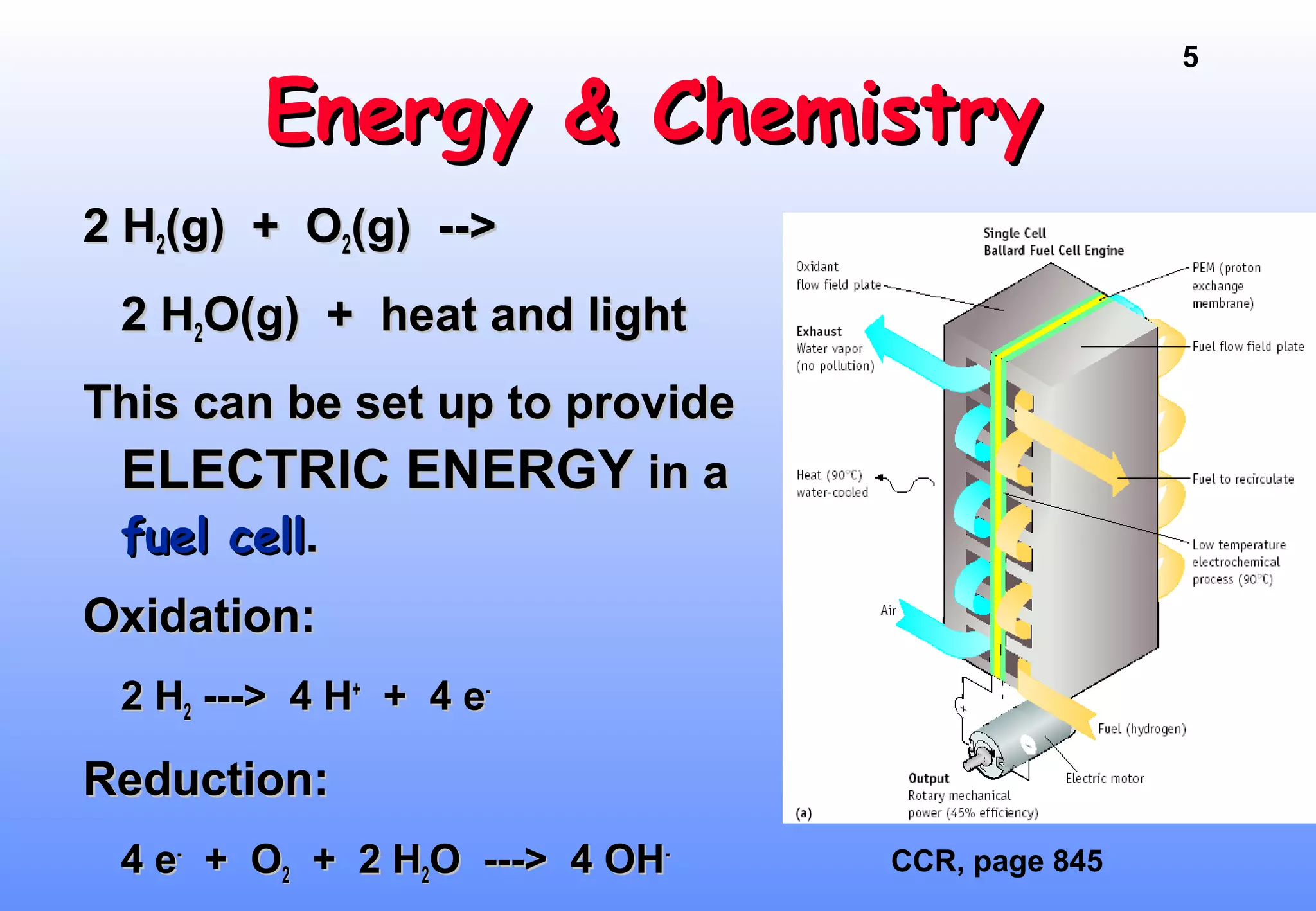
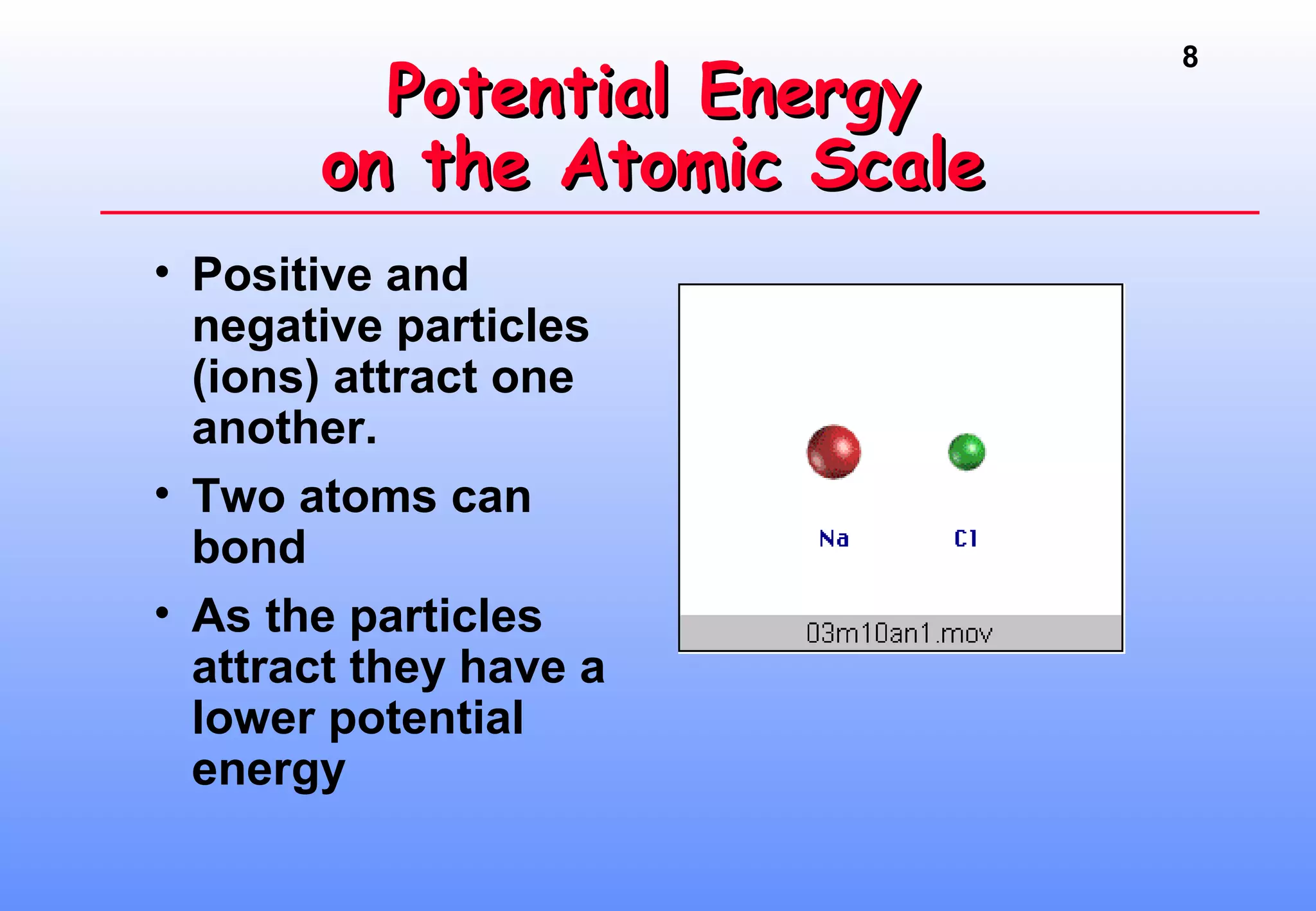
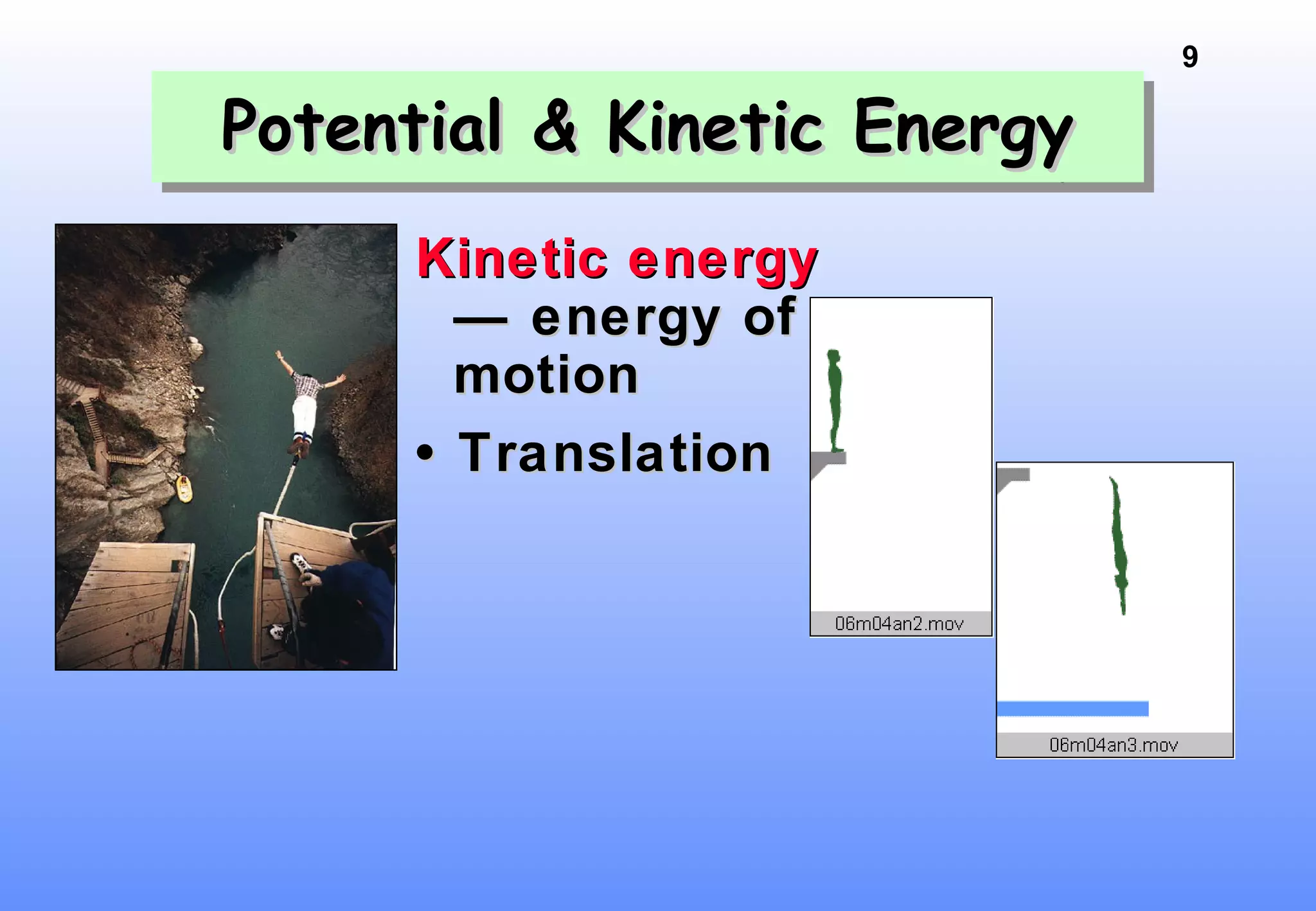
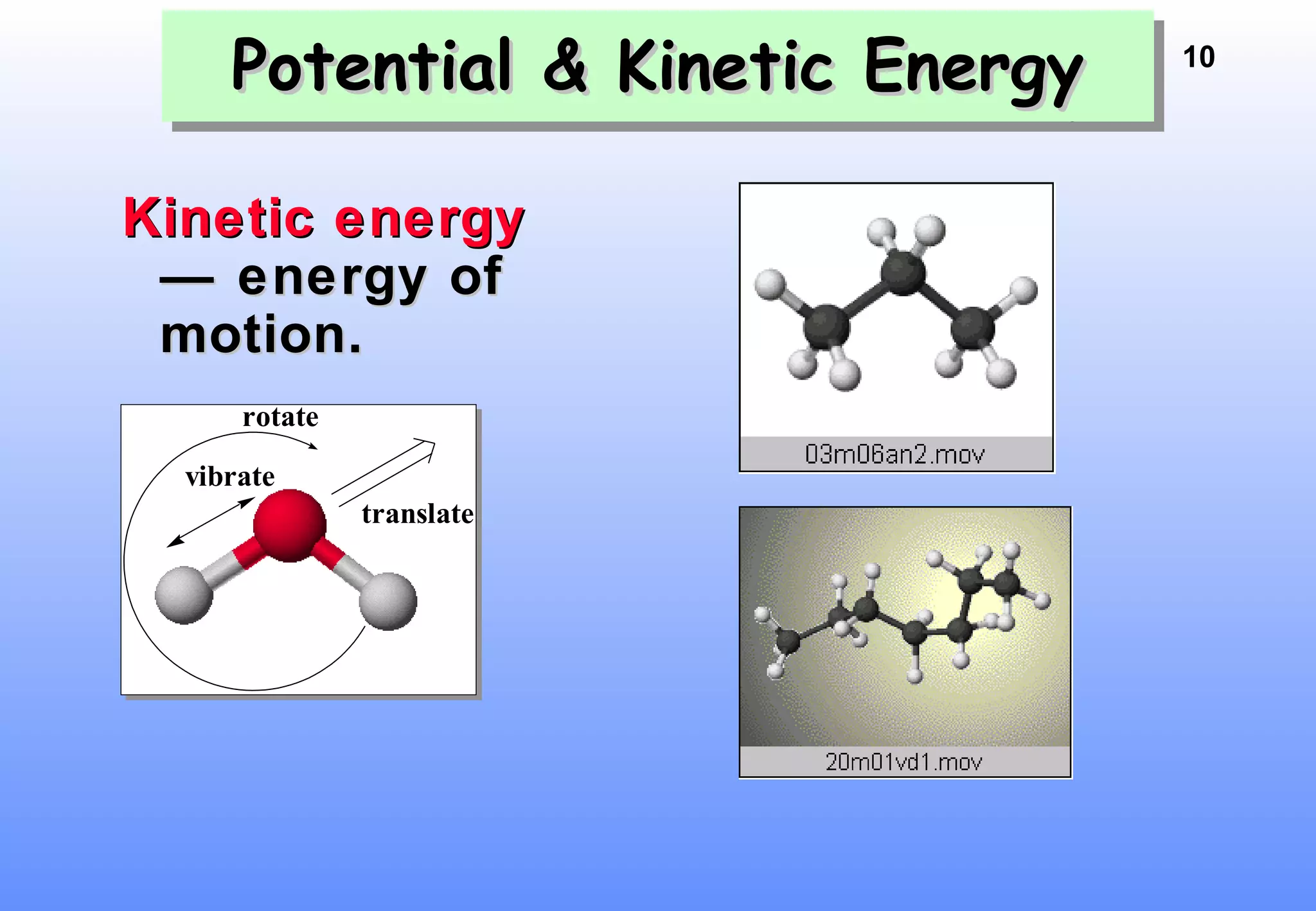
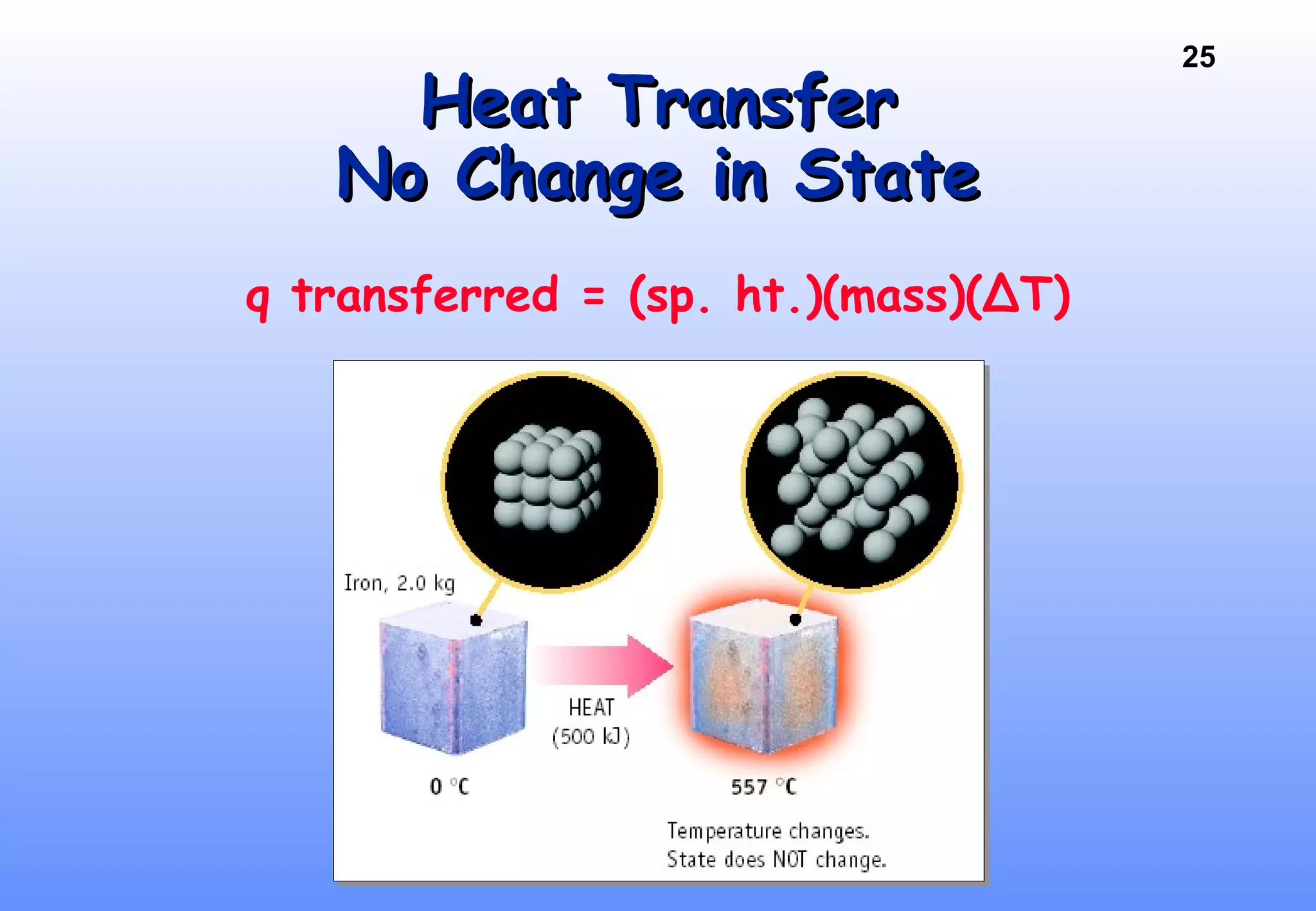
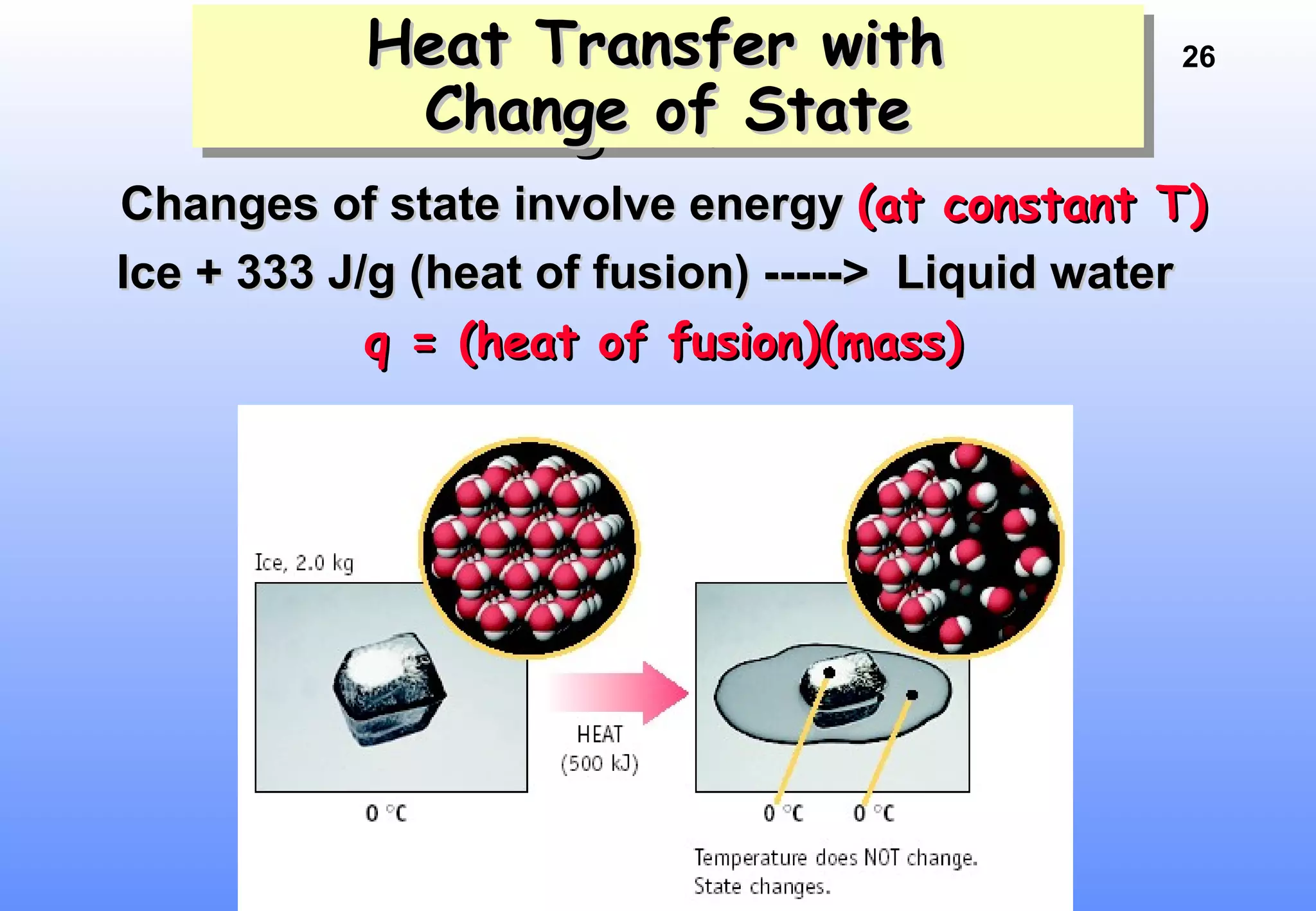
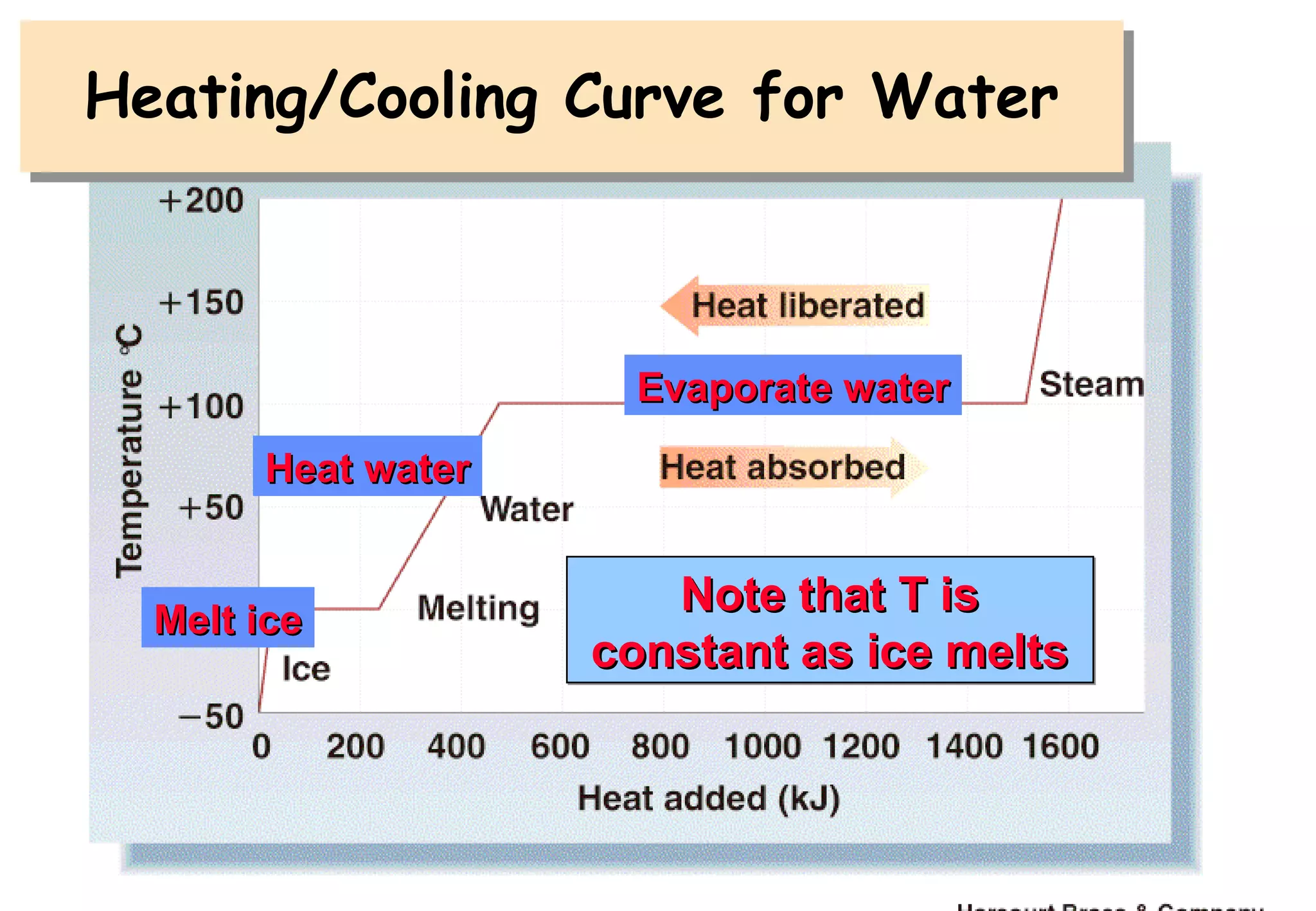
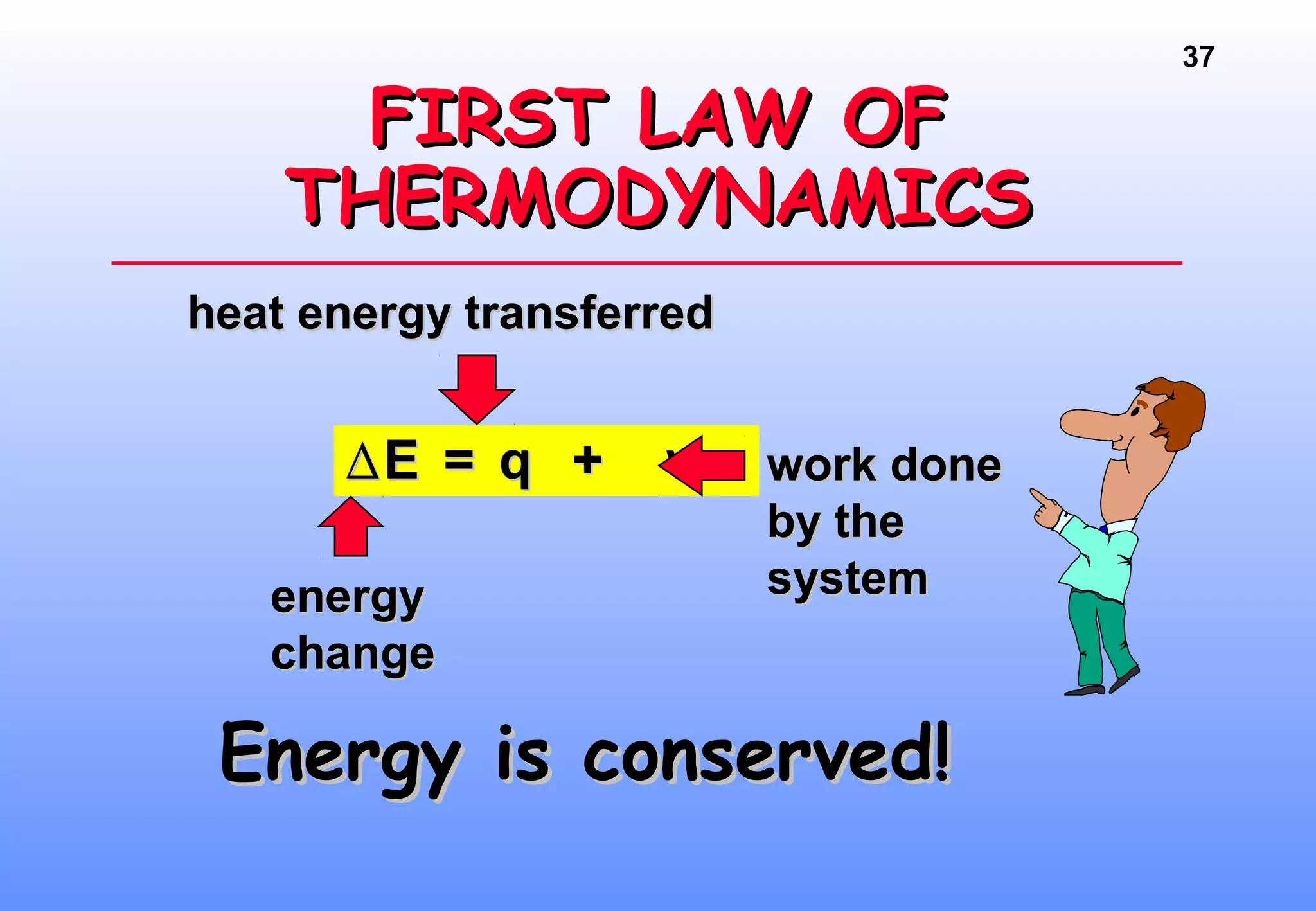
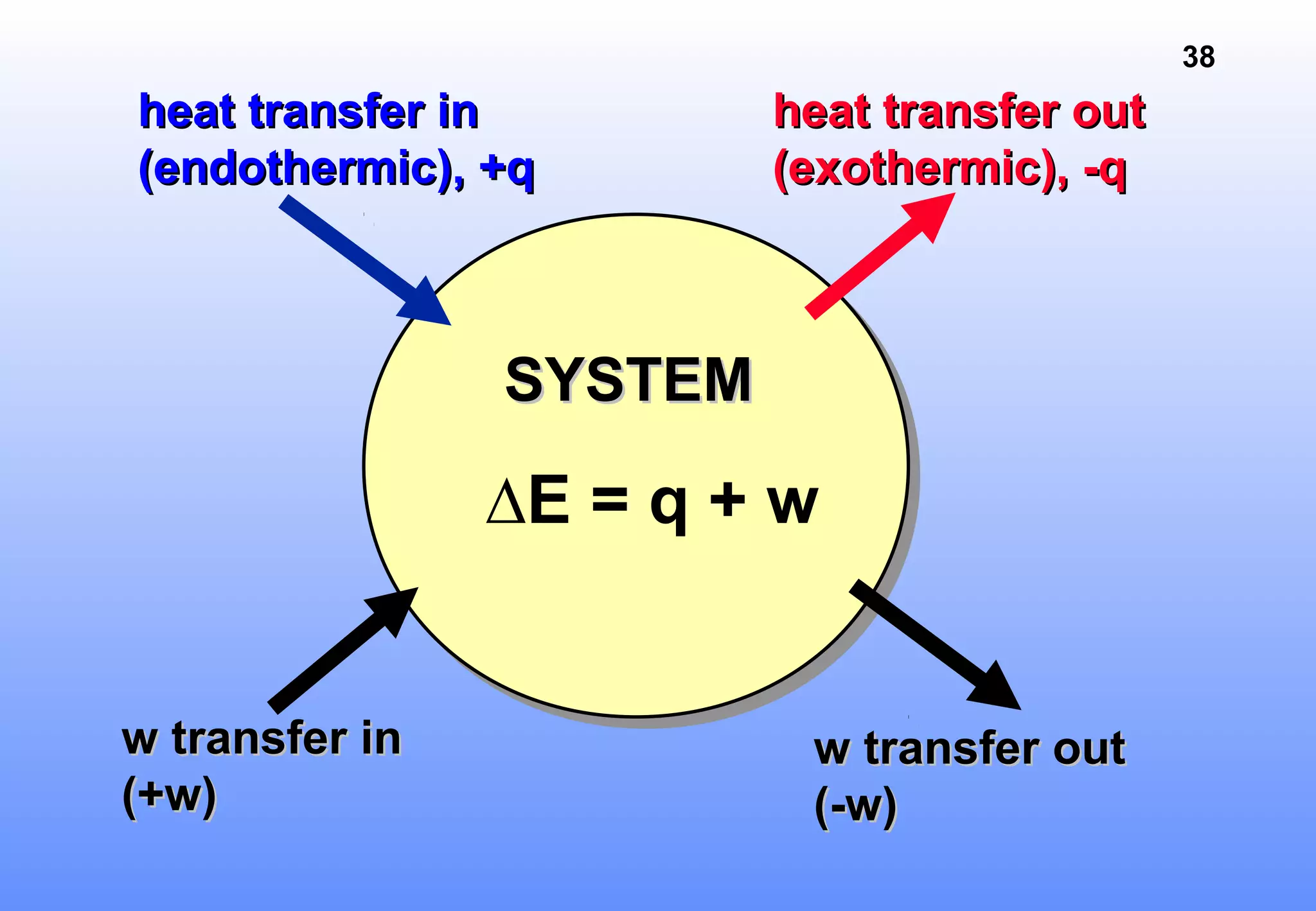
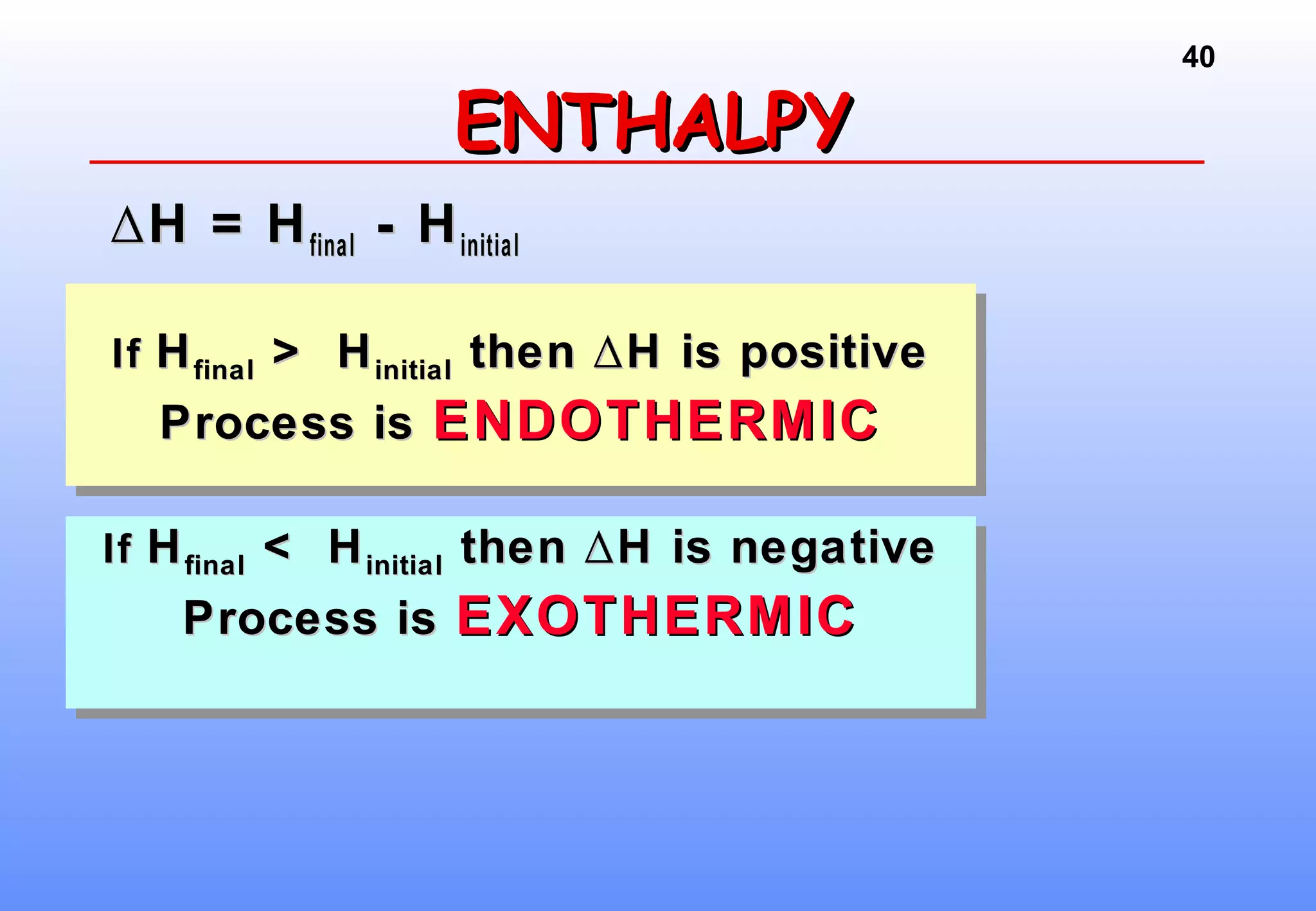
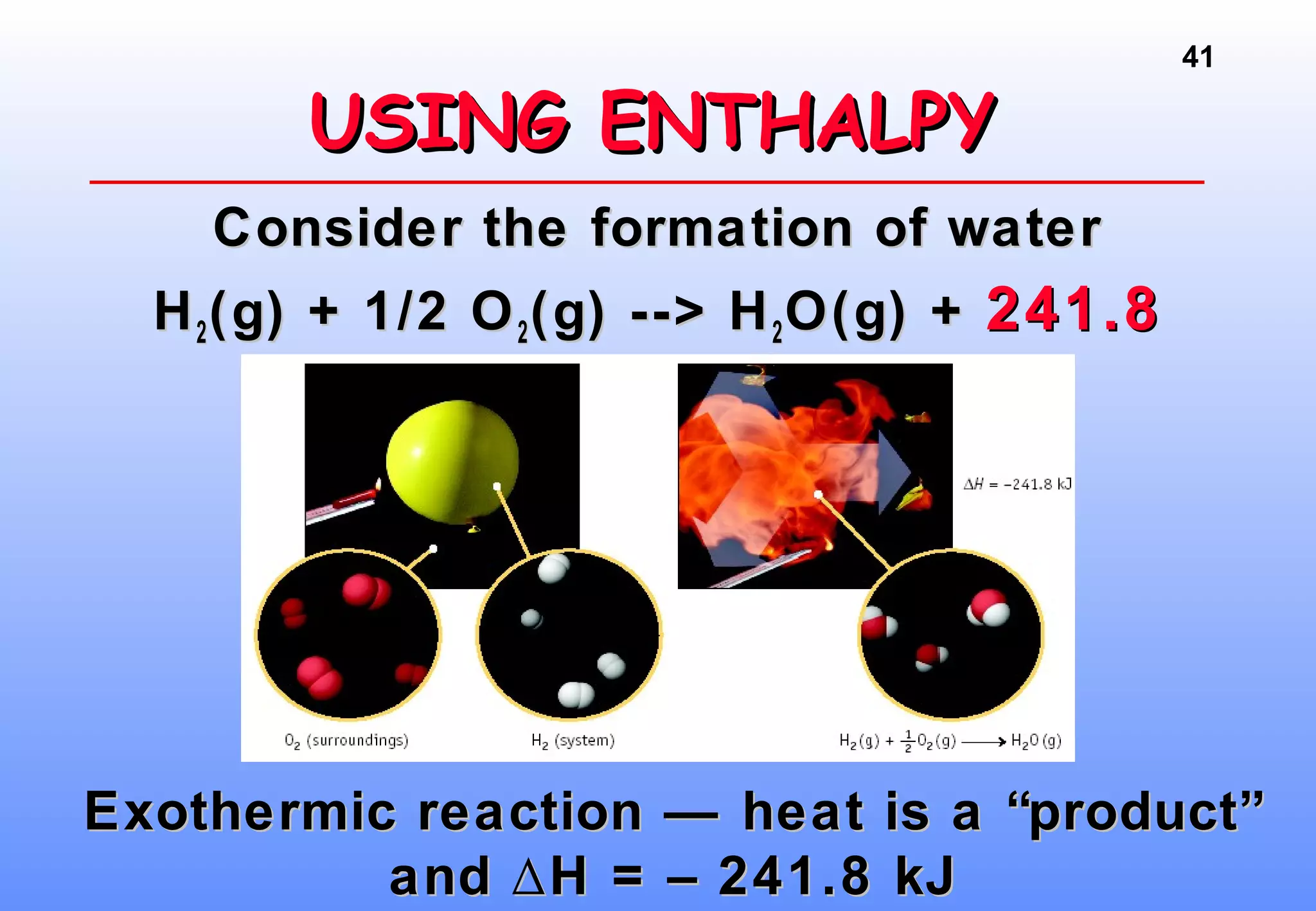
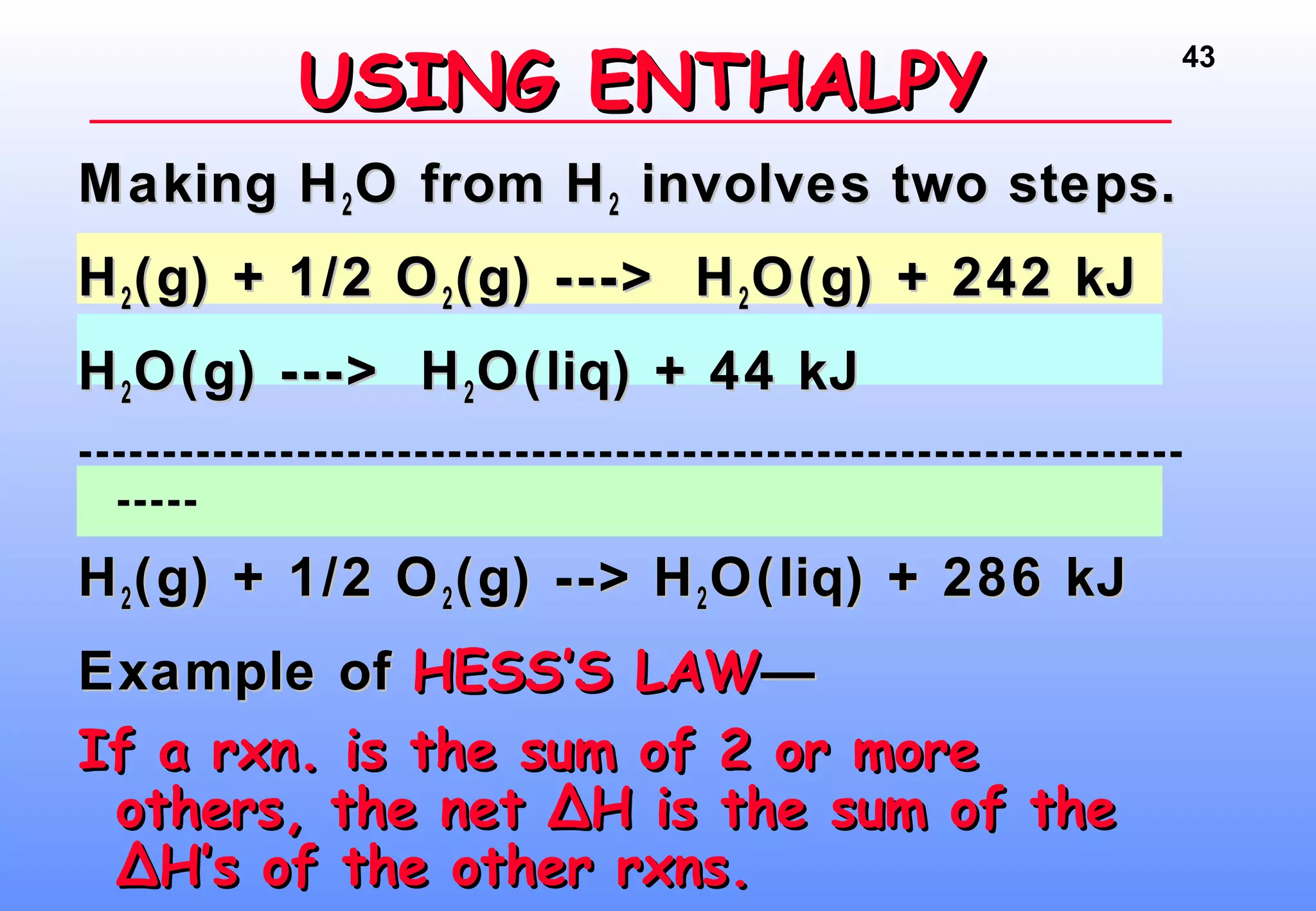
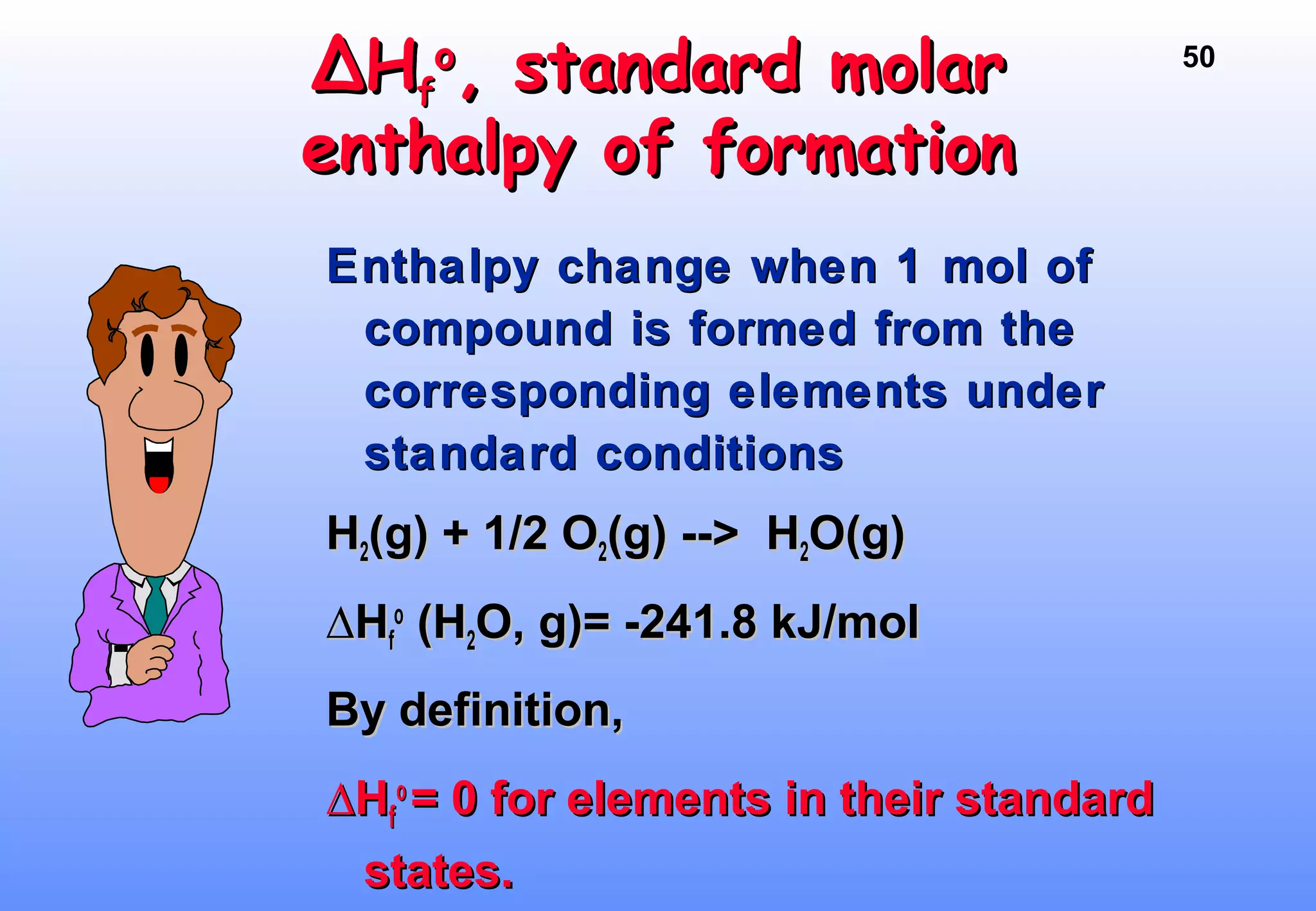
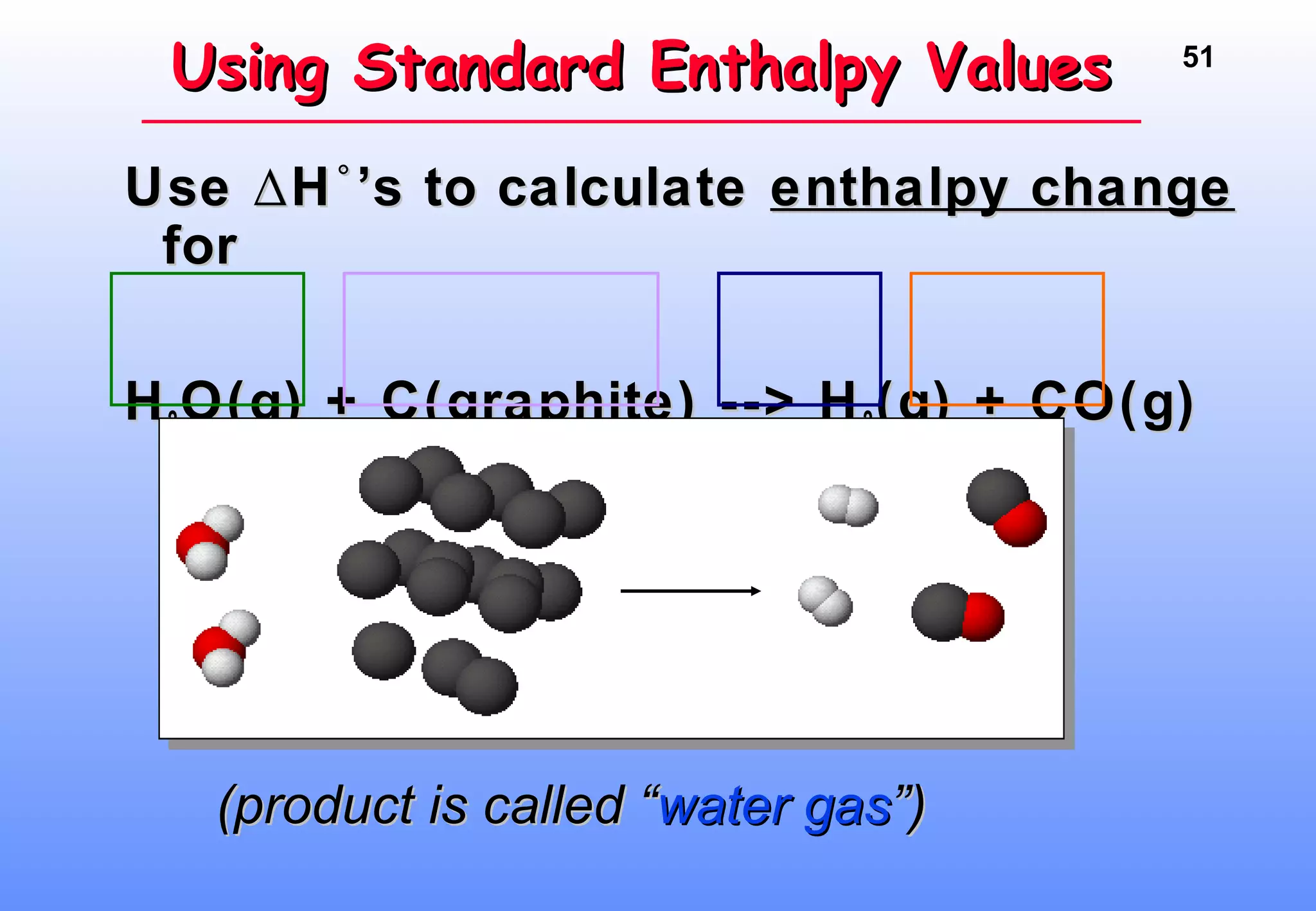
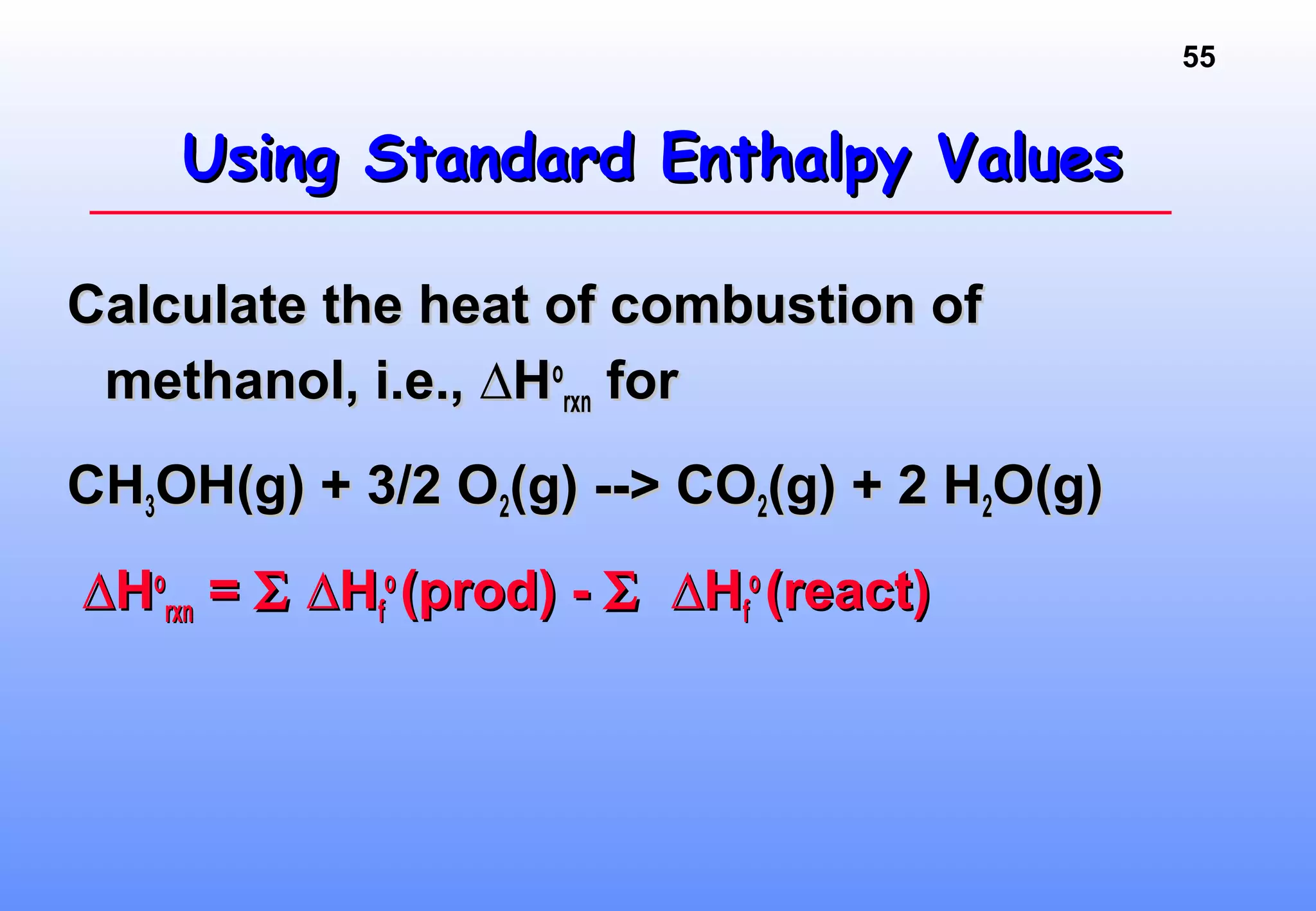
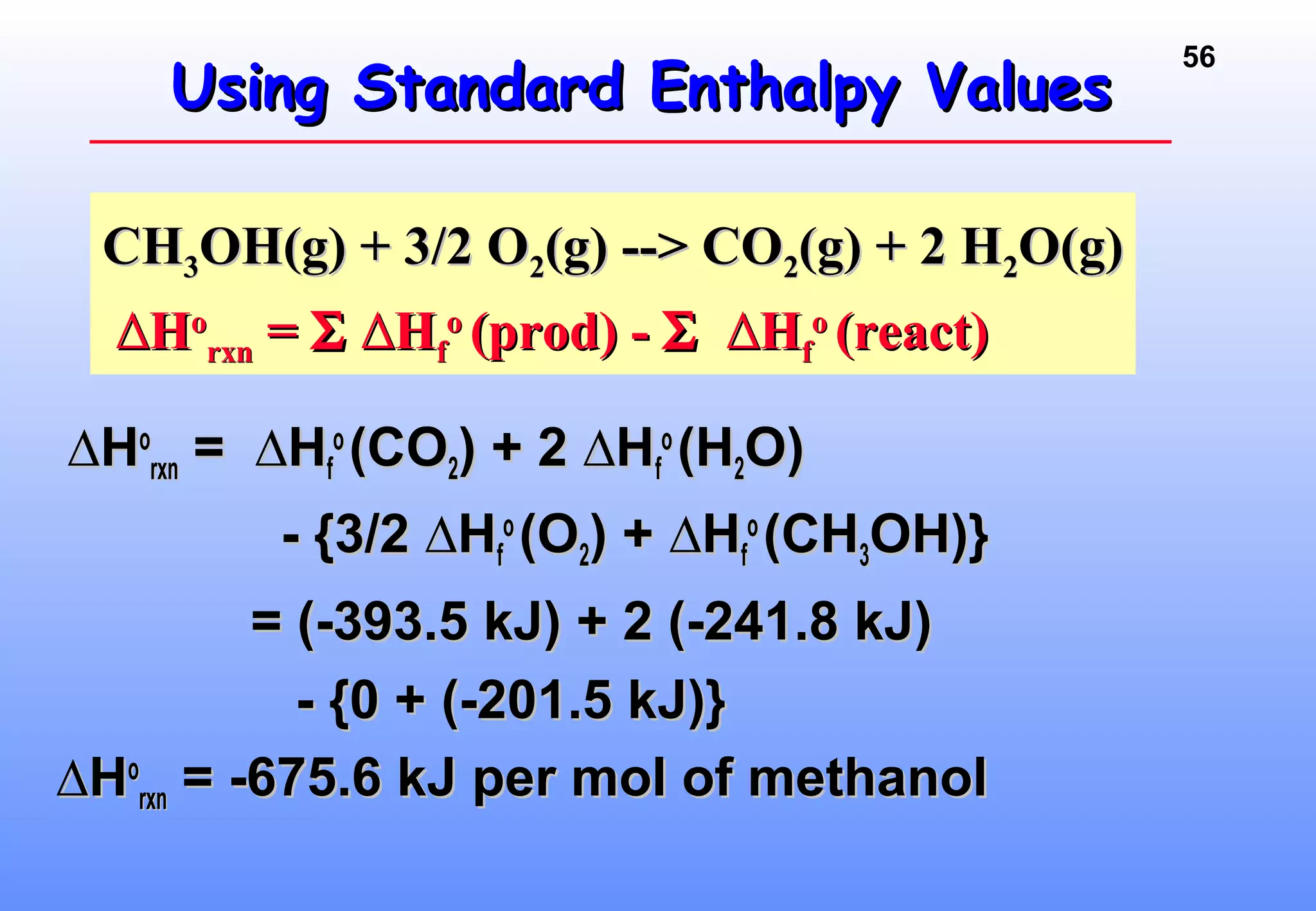
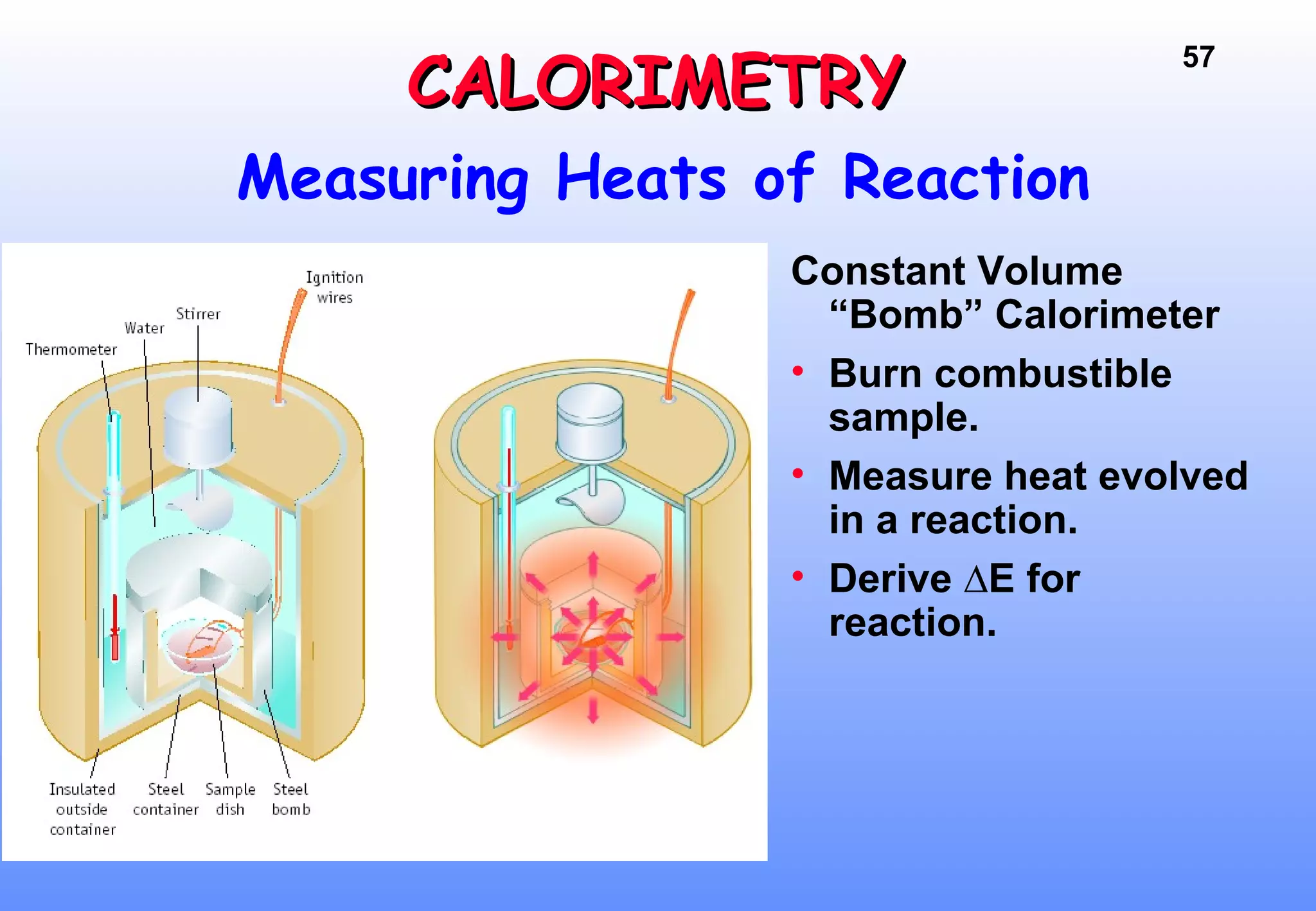
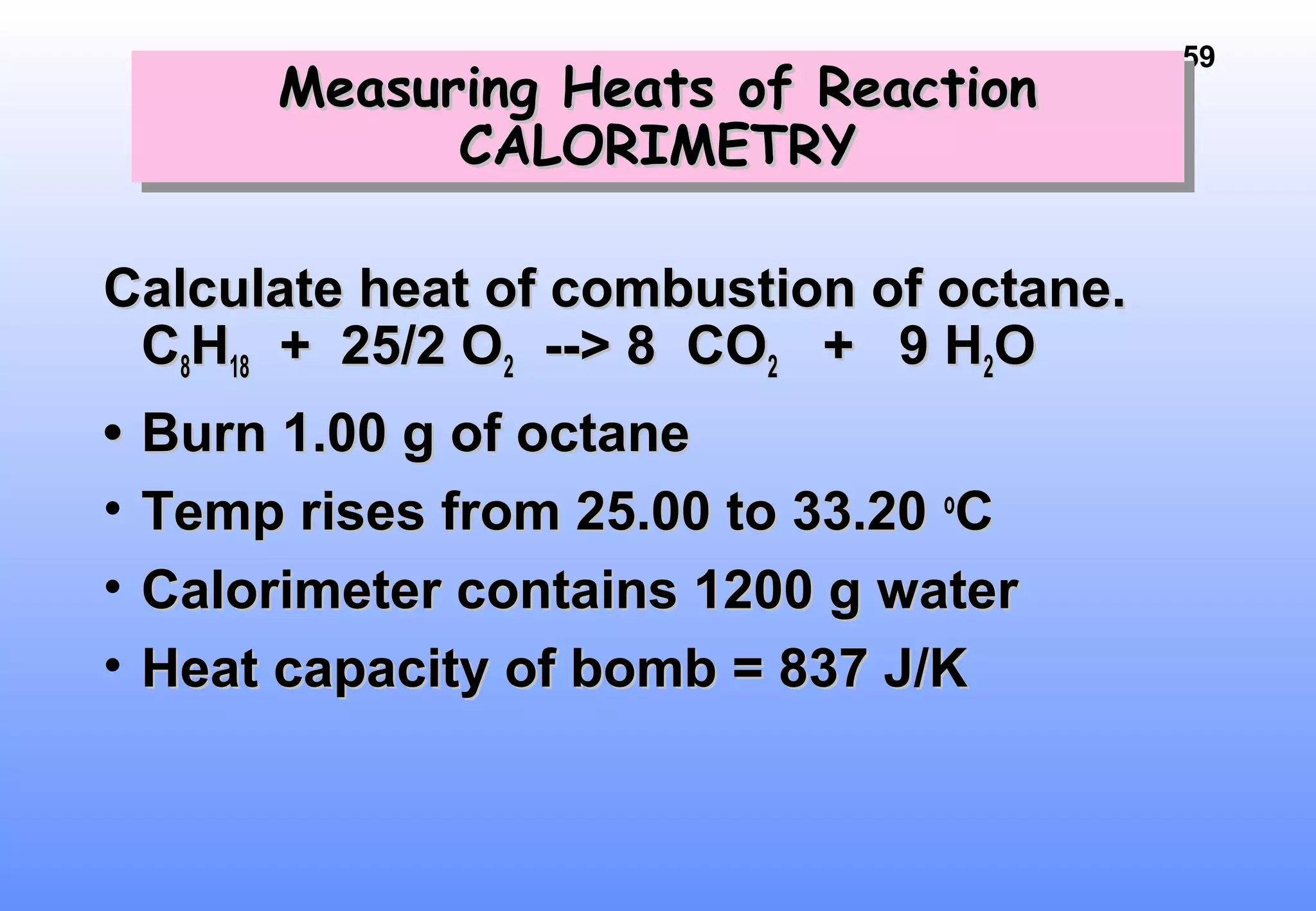
Thermodynamics is the study of heat and work, and state functions. Energy exists in many forms including heat, light, electrical, kinetic, and potential. Heat is the transfer of energy between objects due to a temperature difference. Chemical reactions are driven by thermodynamics and kinetics. Reactions that transfer energy to the surroundings are product-favored. The first law of thermodynamics states that the change in energy of a system equals heat transferred plus work done. Enthalpy represents the heat transferred at constant pressure for a chemical reaction.