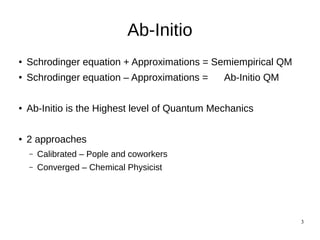
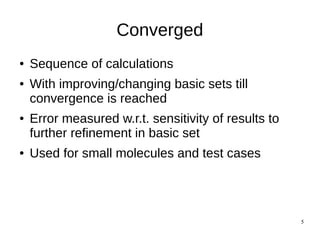
The document discusses quantum mechanics and ab initio methods. It explains that the Schrodinger equation is the basic wave equation used in quantum mechanics to describe particle behavior. Ab initio methods refer to solving the Schrodinger equation without approximations, which is the highest level of quantum mechanics. There are two approaches to ab initio - calibrated uses a fixed basis set and calibrates calculations, while converged uses improving basis sets until results converge. Ab initio is based on the self-consistent field method and Hartree-Fock approximation to calculate energies and new orbitals iteratively until self-consistency is achieved.