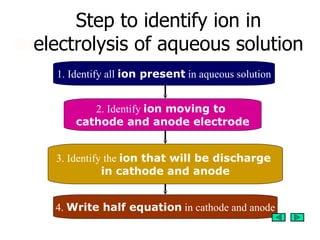
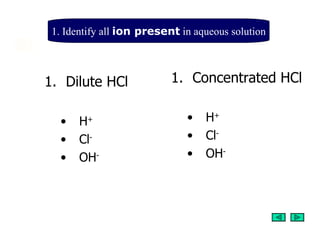
1. The document discusses the steps to predict the products of electrolysis of aqueous solutions by identifying the ions present, which ions move to the cathode and anode, and which ions will discharge at each electrode.
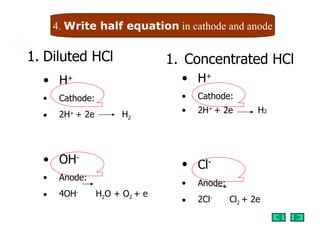
2. Half reactions are written for the electrolysis of diluted and concentrated hydrochloric acid that show hydrogen ions discharging at the cathode and chlorine or oxygen discharging at the anode.
3. Observations are described for experiments electrolyzing diluted and concentrated hydrochloric acid that are consistent with the predicted half reactions.