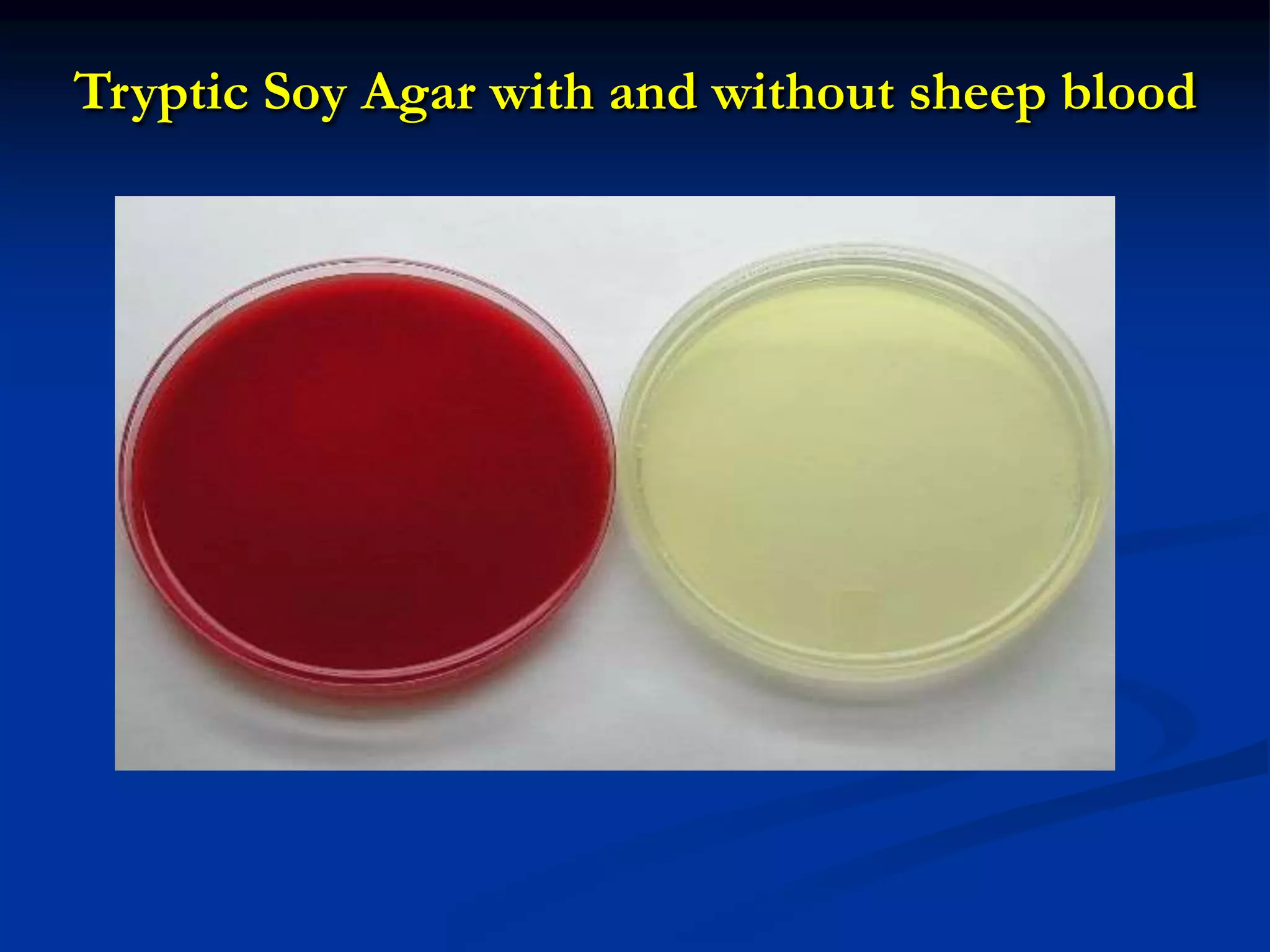
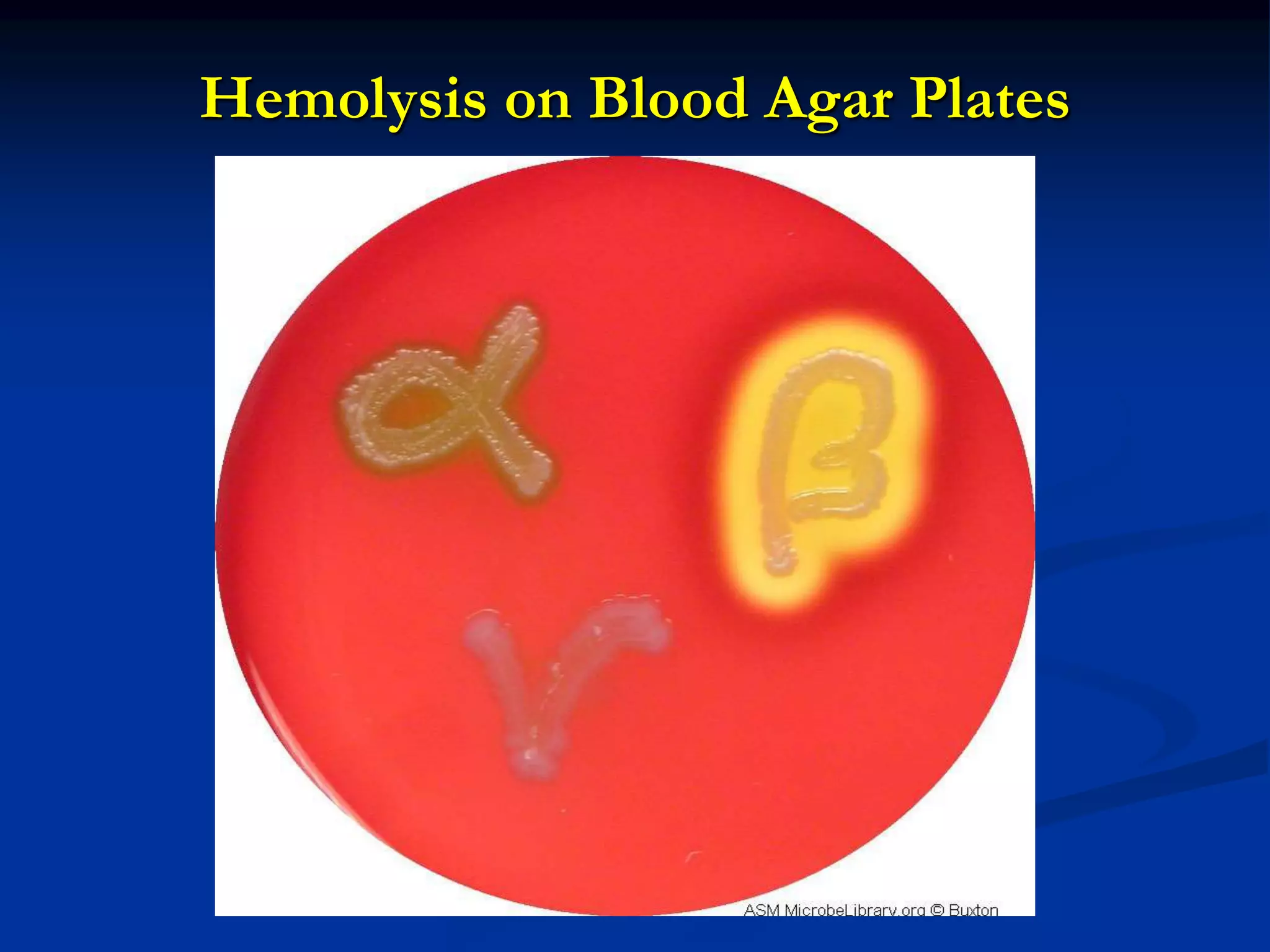
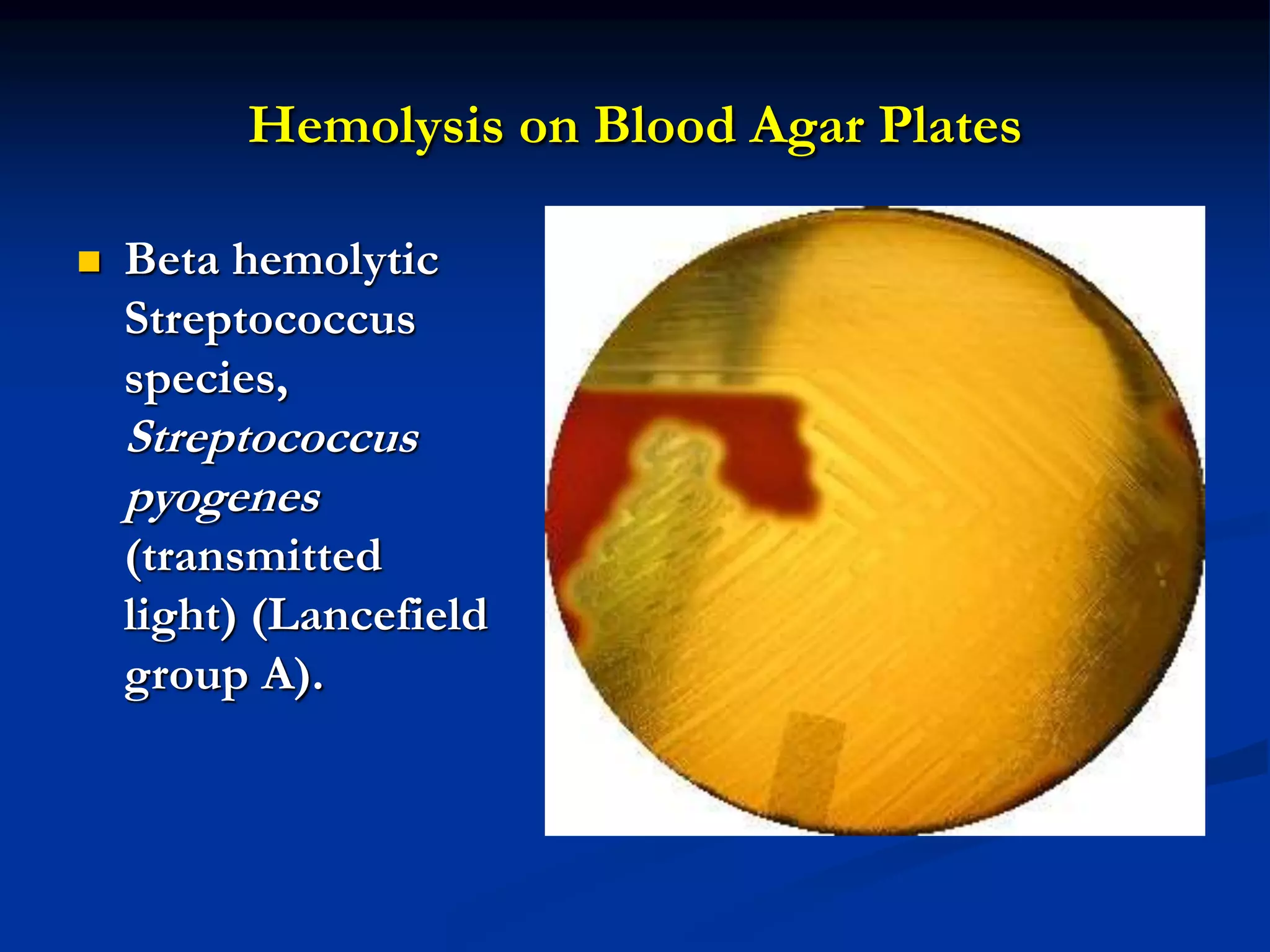
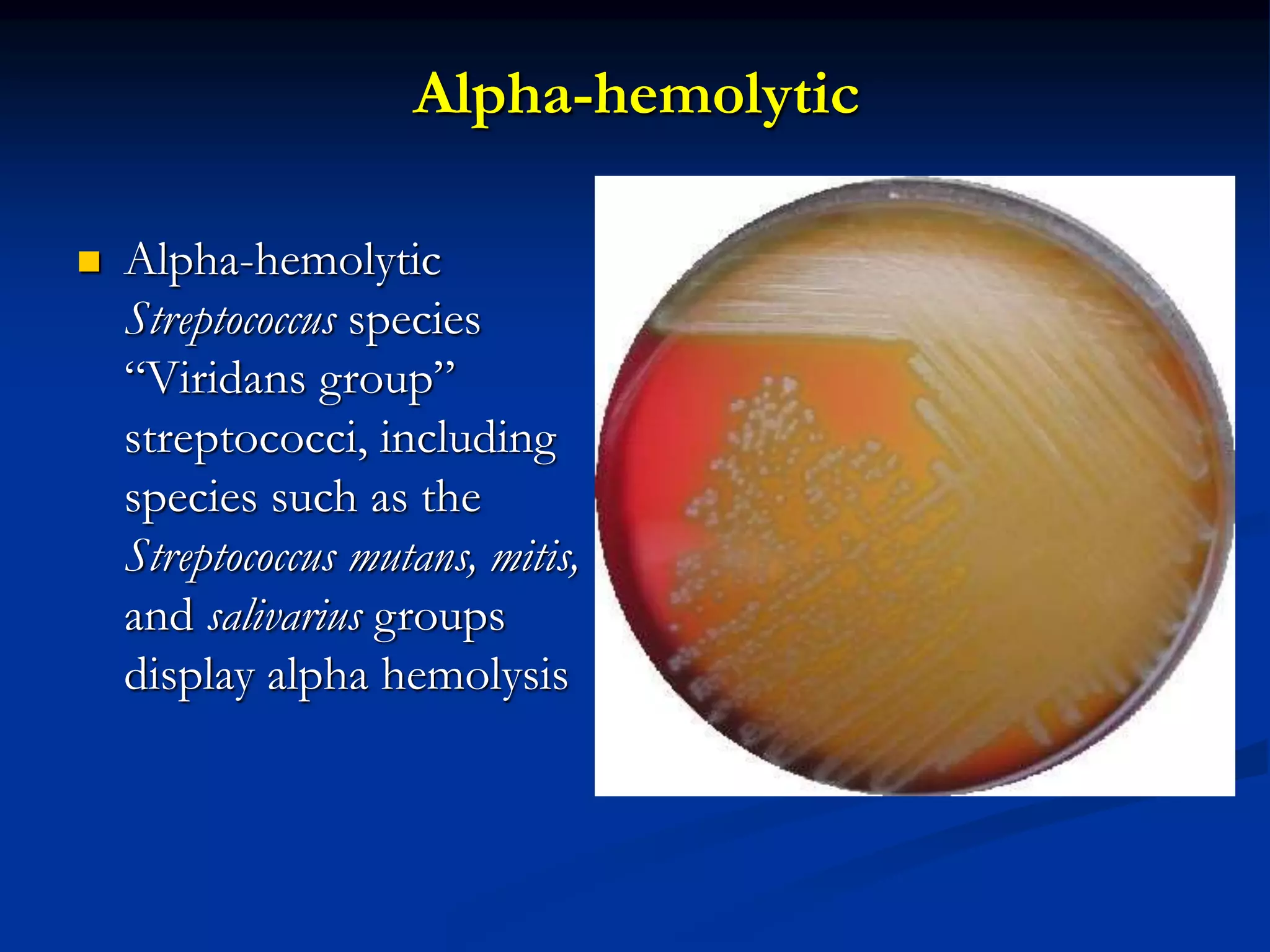
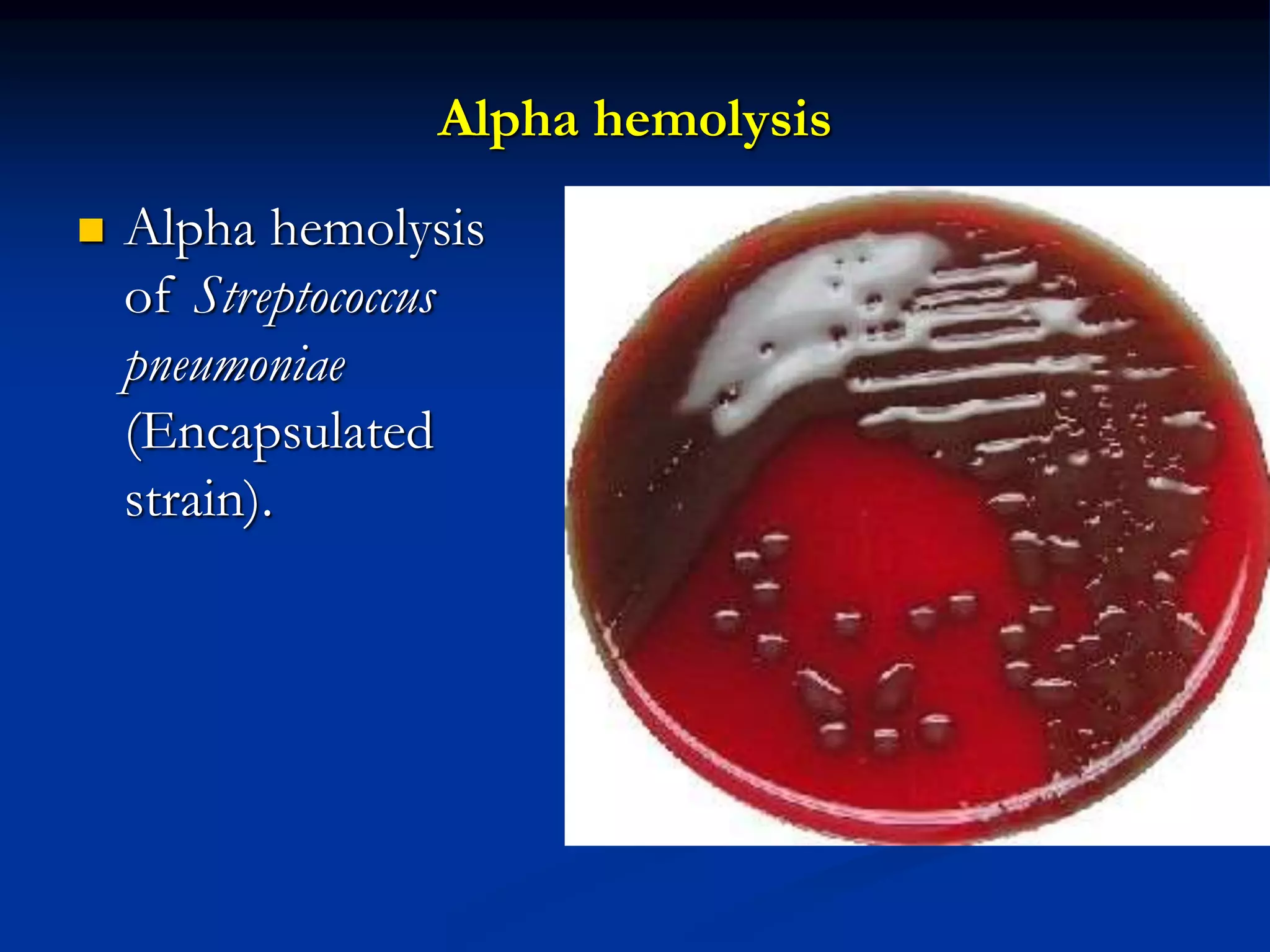
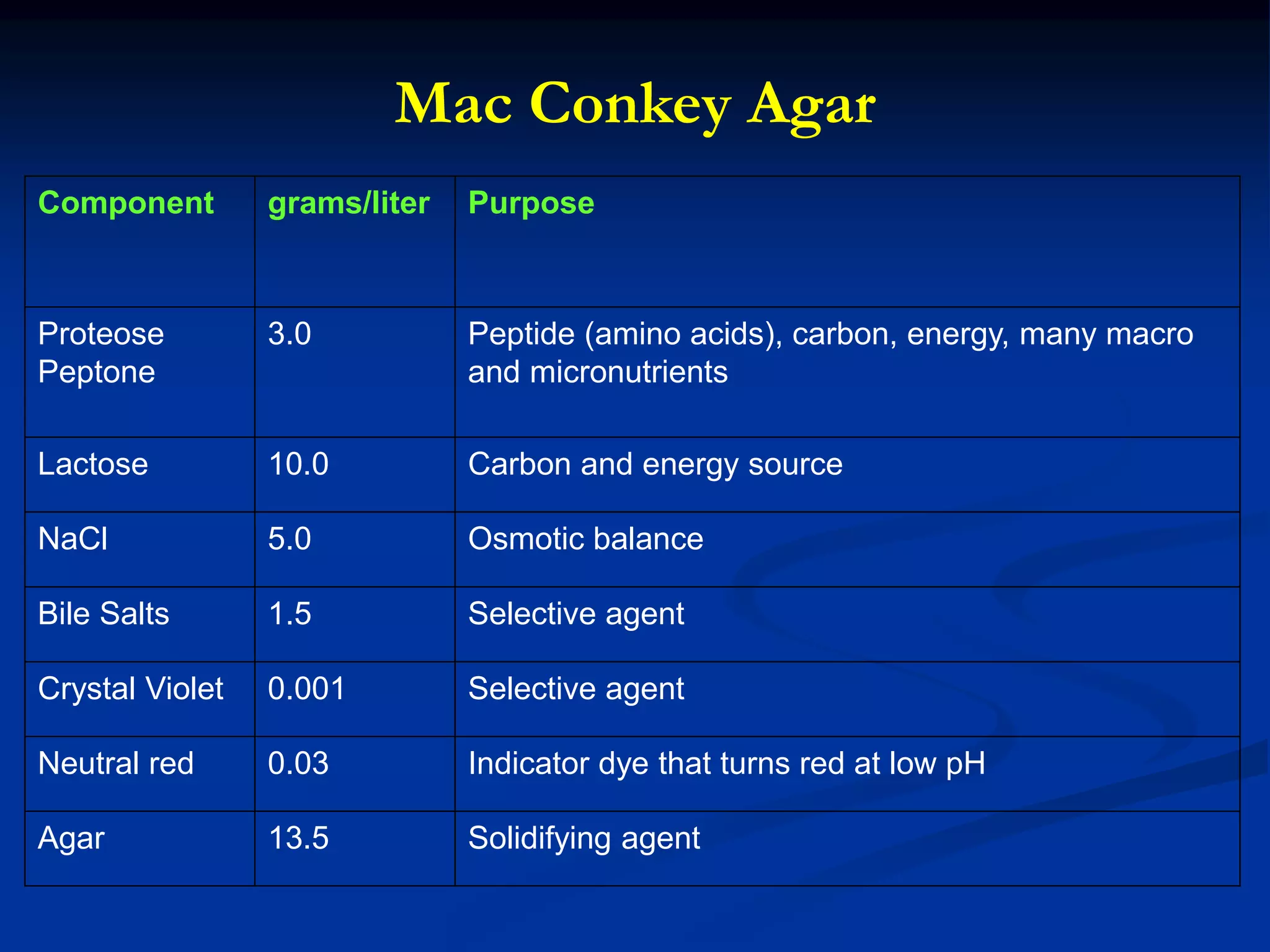
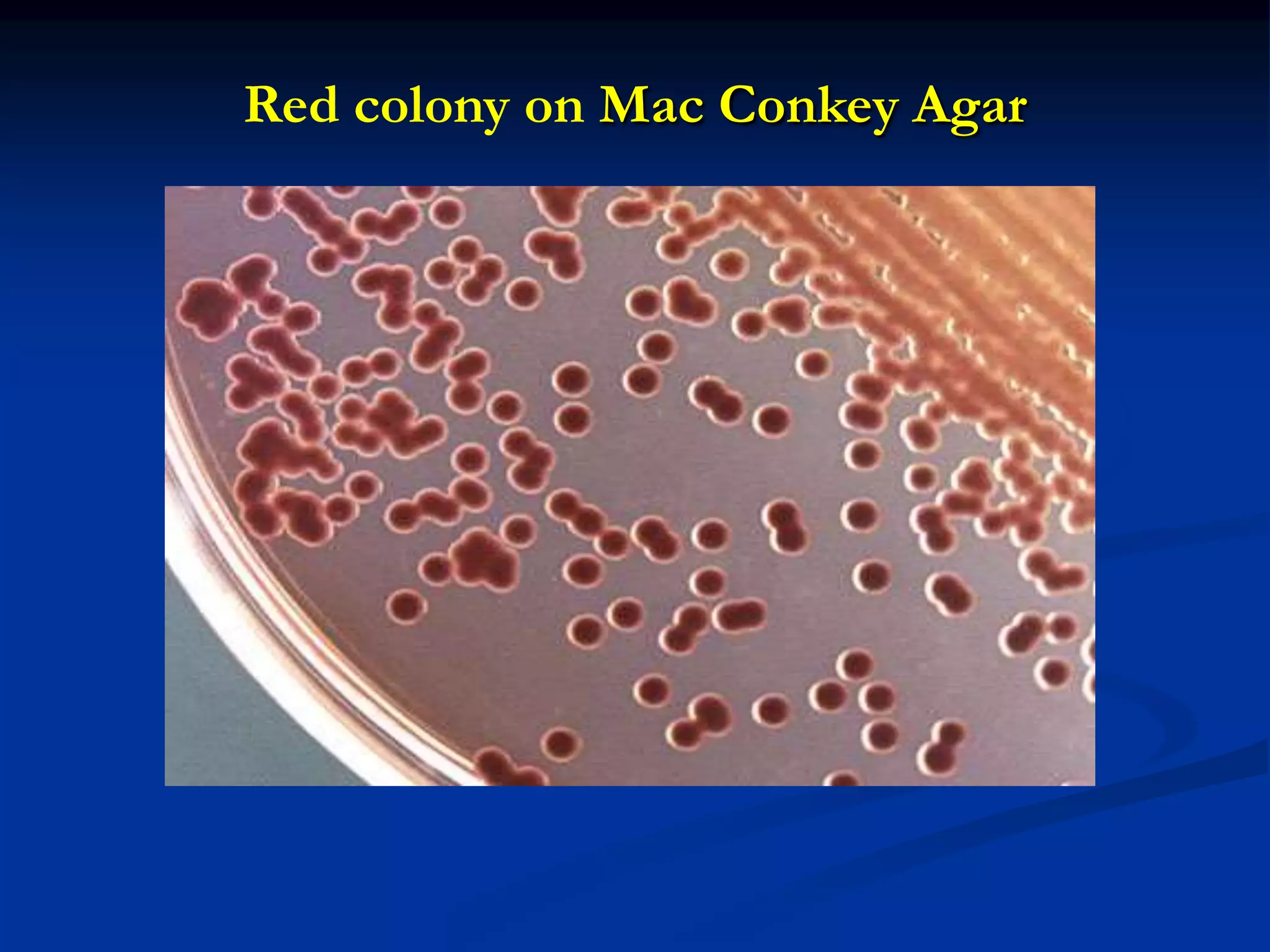
The document describes different types of media used to culture and differentiate bacteria, including blood agar, MacConkey agar, and techniques for colony isolation and examination. Blood agar is used to detect hemolysis (lysis of red blood cells) around bacterial colonies. There are three types of hemolysis - alpha, beta, and gamma. MacConkey agar selects for gram-negative bacteria and differentiates lactose fermenters from non-fermenters. Colony morphology provides clues to bacterial identification based on characteristics like size, elevation, texture and pigmentation. Proper isolation techniques like streaking or spreading are used to obtain isolated colonies from mixed cultures.