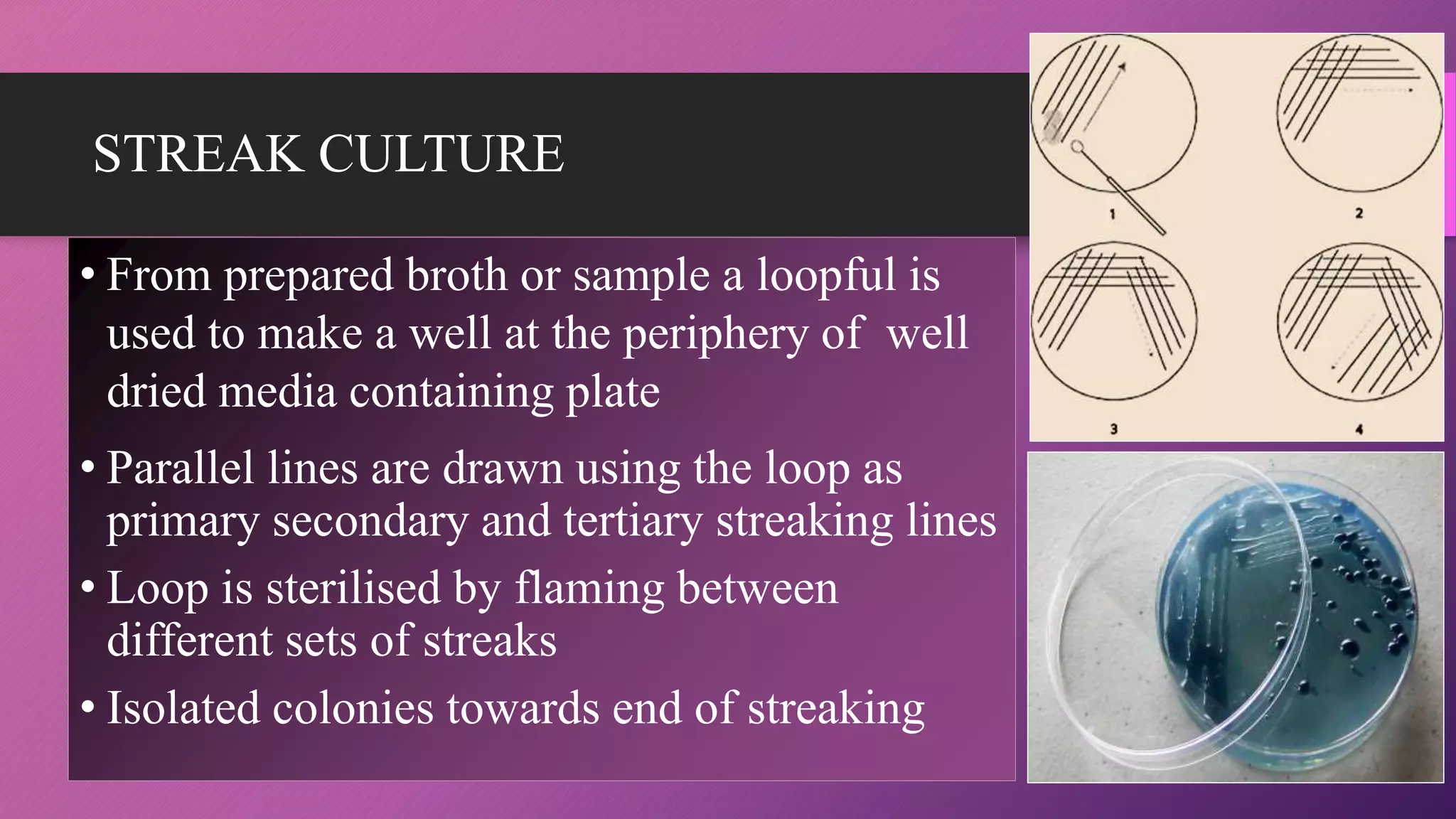
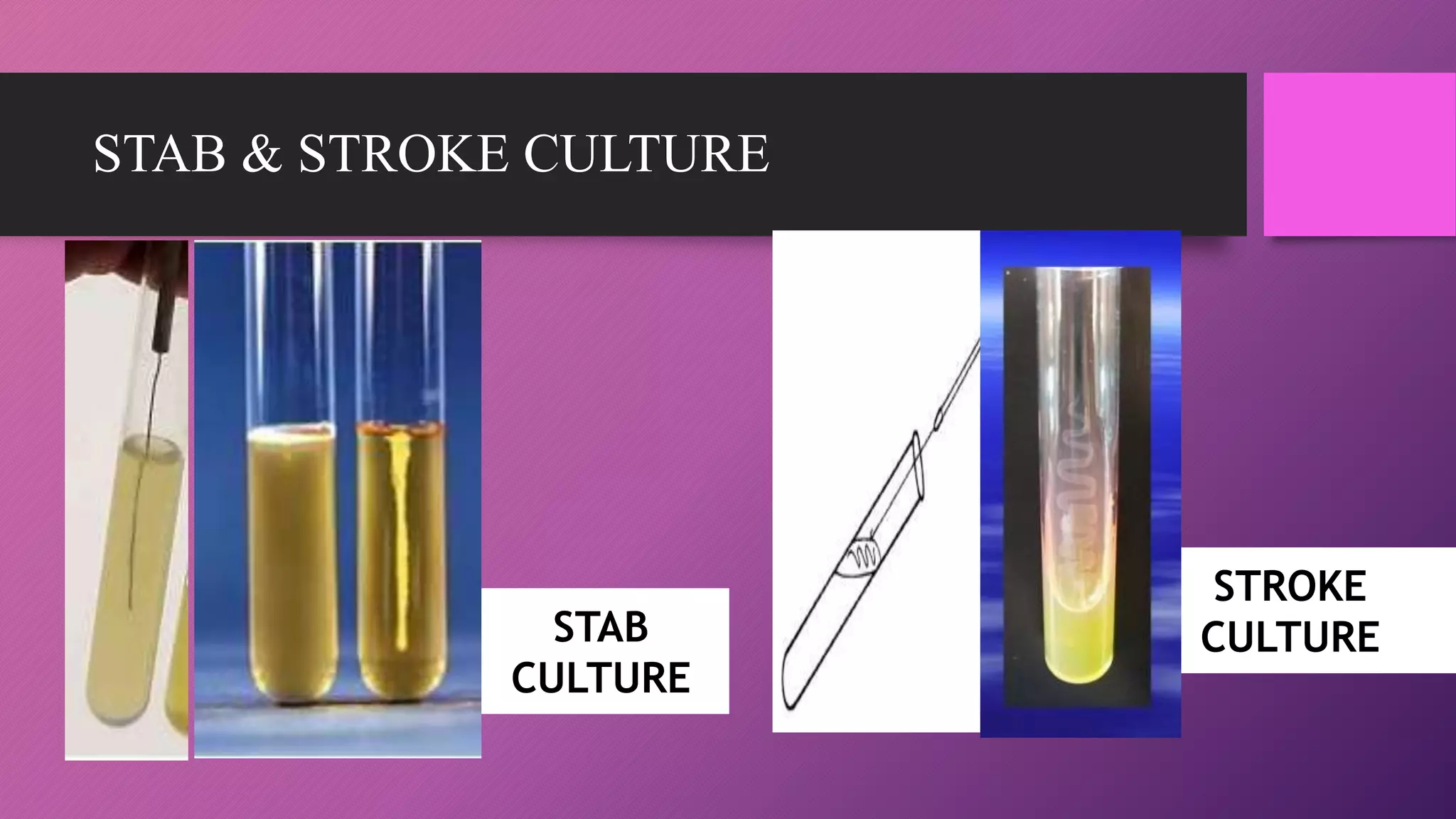
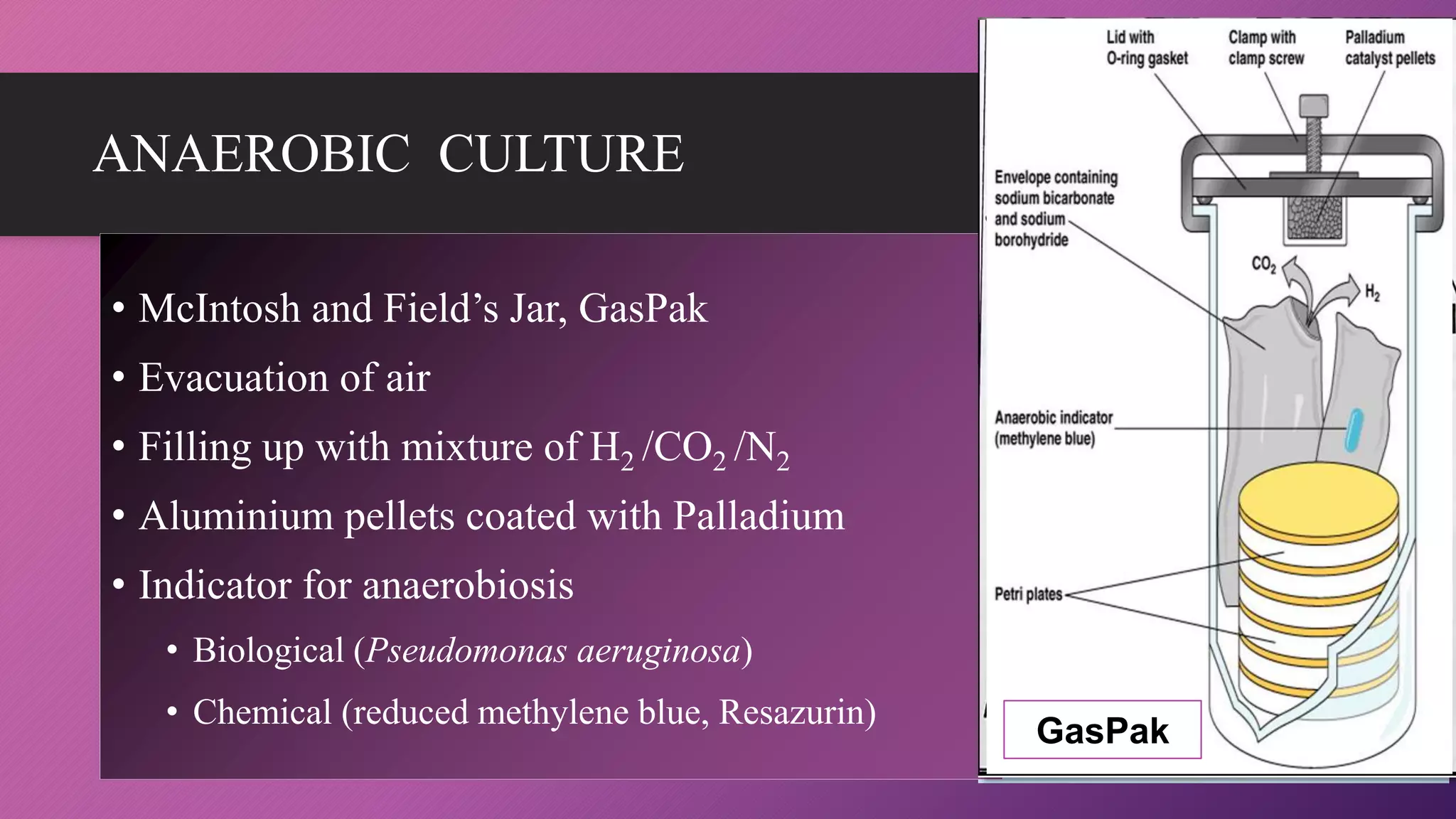
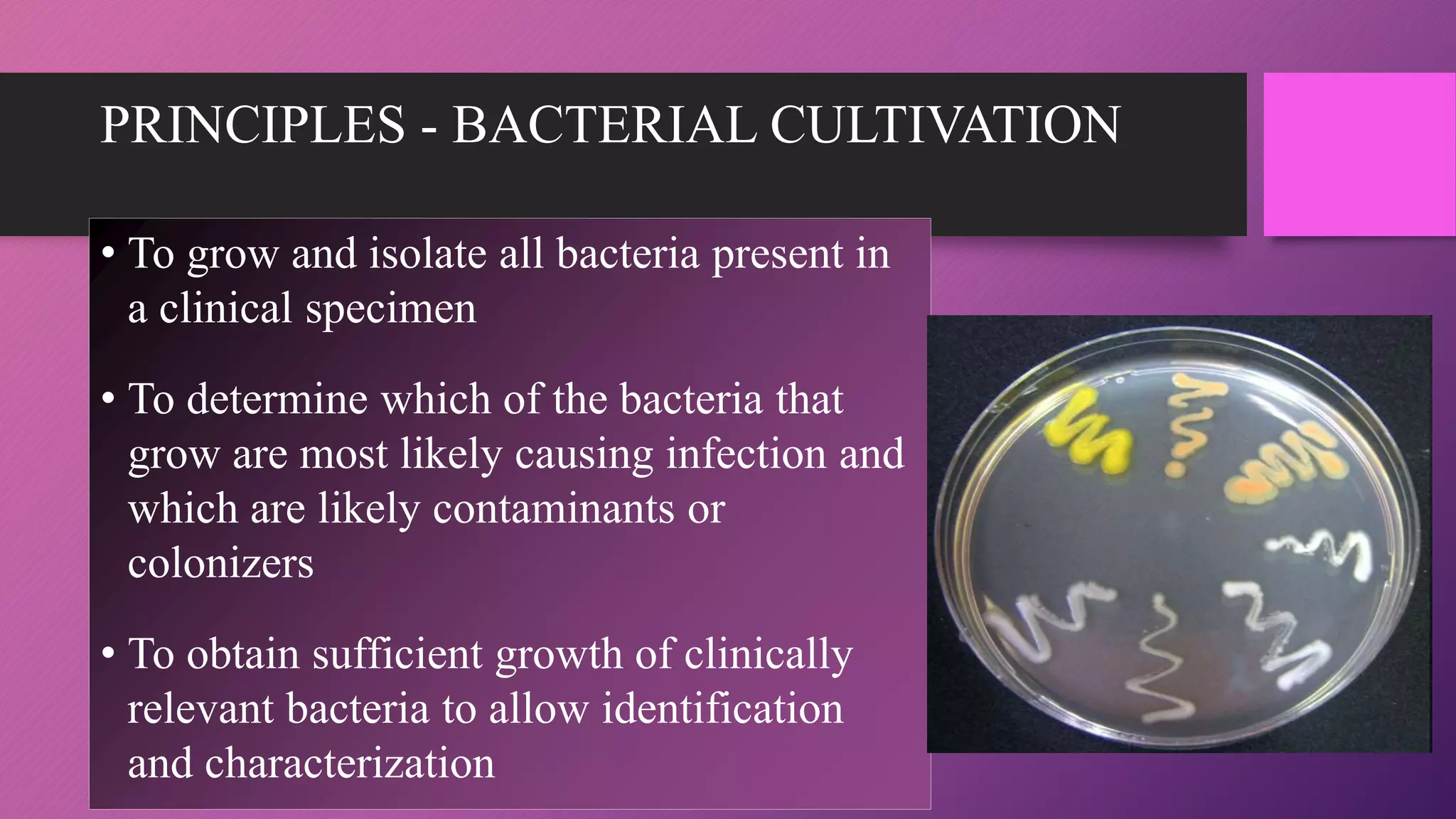
The document provides an overview of bacterial culture methods used for identification and characterization in clinical settings. It discusses various types of culture media, including solid, liquid, and enriched media, as well as specific examples and their applications in isolating pathogenic bacteria. The text also details different culture techniques such as streak, lawn, stab, and pour plate methods, emphasizing their significance in microbiological studies.







![SIMPLE MEDIA / BASAL MEDIA
Most commonly used in routine diagnostic laboratories
Eg: Nutrient Broth, Nutrient Agar
• Peptone Water = Peptone (1%)+ NaCl (0.5%)+ Water
• Nutrient Broth = peptone water + meat extract
• Nutrient agar = NB + 2% agar
[Semi-solid medium = Agar conc. Reduced (0.2 - 0.5%) ]
Glucose Broth = NB + 0.5% Glucose](https://image.slidesharecdn.com/culturemediaandmethods-200205104033/75/Culture-media-and-Culture-methods-10-2048.jpg)