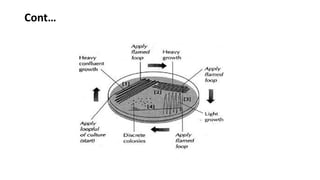
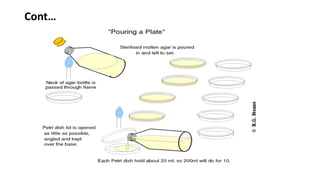
Culture media provides nutrients for bacteria to grow in vitro. Agar is commonly used to solidify liquid media into solid media, allowing for distinct bacterial colonies to form. Specialized media can be selective, differential, or enriched depending on the bacteria being cultured. Proper preparation, storage, and pouring techniques are required to avoid contamination when using culture media in the microbiology laboratory.