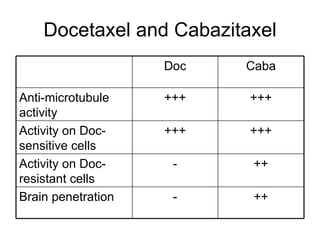
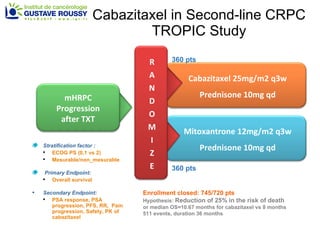
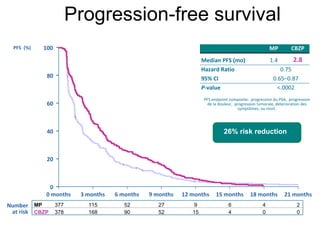
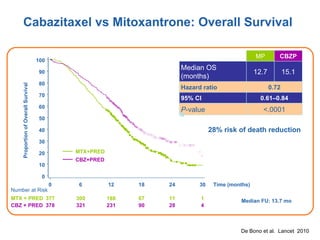
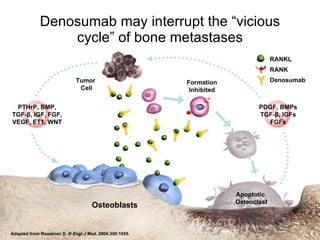
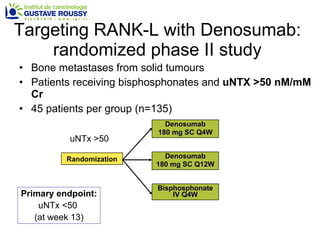
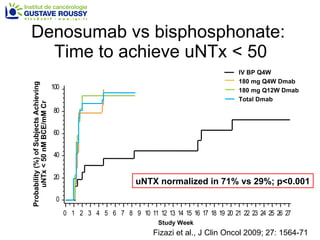
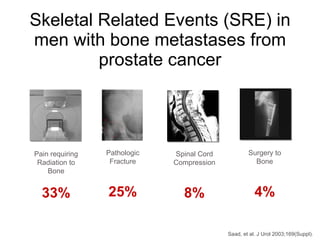
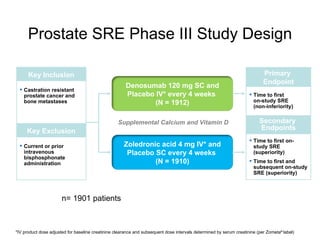
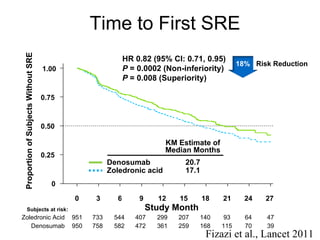
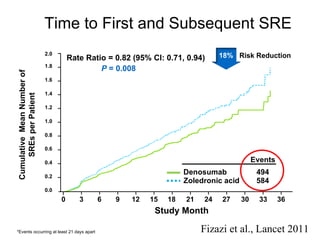
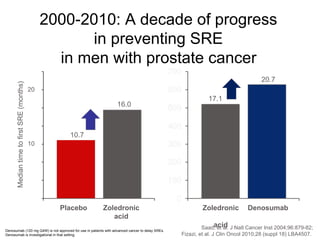
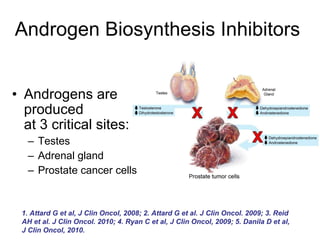
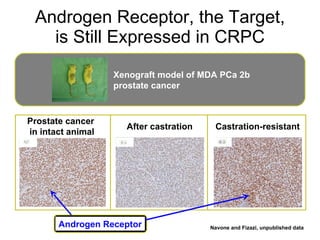
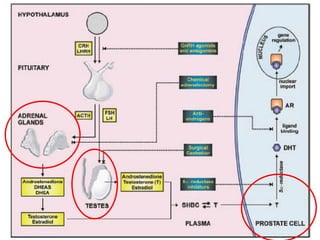
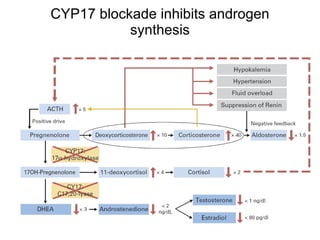
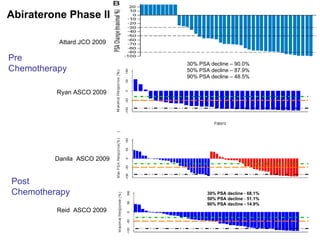
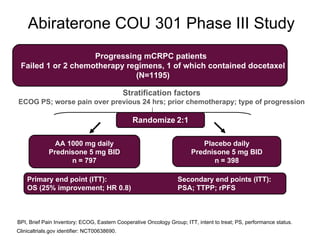
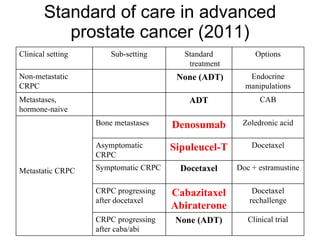
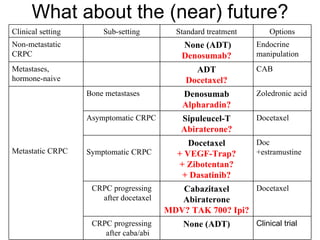
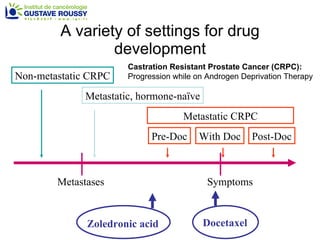
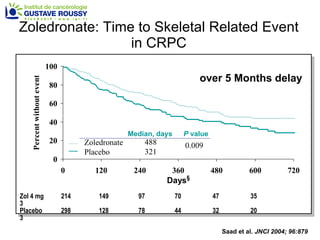
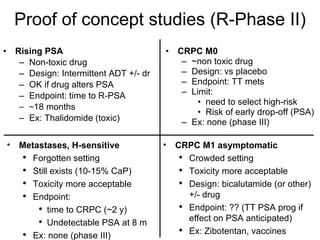
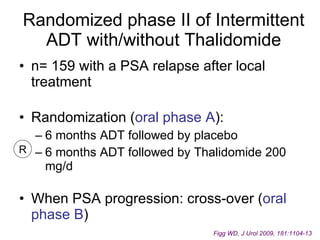
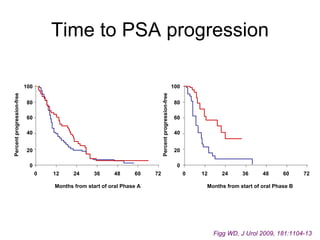
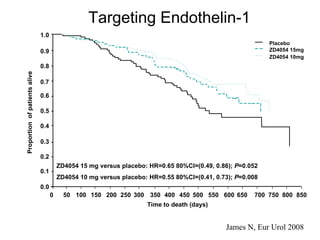
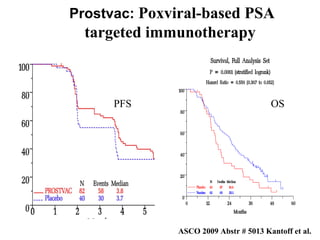
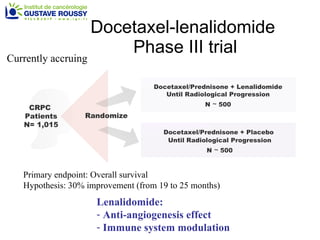
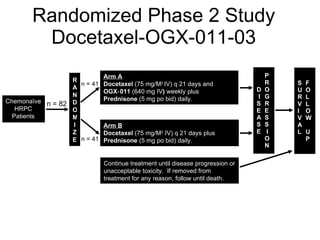
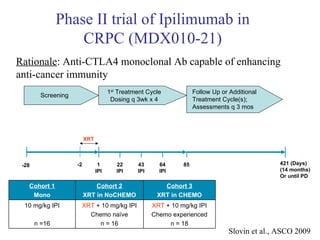
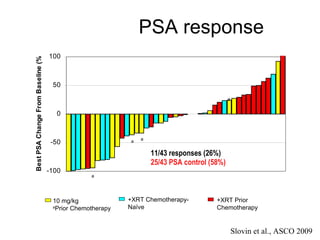
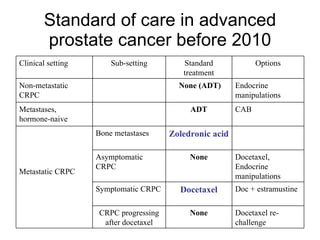
The document discusses drug development in prostate cancer, including settings for clinical trials and endpoints accepted by regulatory agencies. It provides examples of both successful and failed phase 2 and 3 clinical trials, demonstrating the challenges of developing effective drugs to treat prostate cancer. Key approvals in 2010 included sipuleucel-T, cabazitaxel, and denosumab, representing progress after many failed drug candidates in prior years.





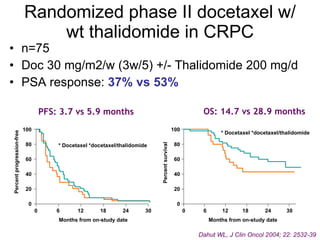
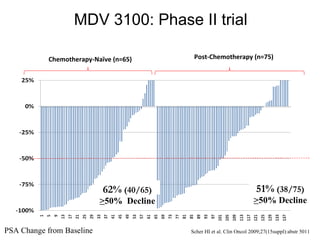
![Median for OGX-011: 23.8 95% C.I. [16.2 - .Inf] Median for Std Trt: 16.9 95% C.I. [12.8 - 25.8] 1 Variables predictive of OS on multivariate analysis: PS 0 vs 1 (P < 0.0001), presence of visceral metastasis (P = 0.01) and treatment assignment Unadjusted HR=0.61 [0.36-1.02], P=0.06 Multivariate analysis 1 HR=0.49 [0.28-0.85], P=0.01 First-Line CRPC Phase 2 Study OGX-011-03: Kaplan-Meier Survival Curves as of April 2009 Median survival times now final with only one patient lost to follow-up prior to 24 months Treatment completed](https://image.slidesharecdn.com/36fizazi-110517094520-phpapp01/85/ECCLU-2011-K-Fizazi-Prostate-cancer-drug-development-19-320.jpg)


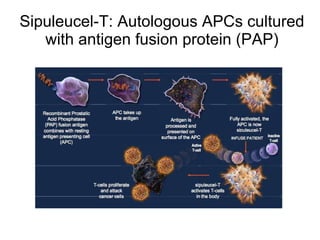
![Sipuleucel-T autologous vaccine: Overall Survival p = 0.032 ( Cox model ) HR = 0.7 8 [95% CI: 0.61, 0.9 8 ] Kantoff PW, NEJM 2010, 363: 411-22 Small EJ, J Clin Oncol 2009; 24: 3089-94 Sipuleucel-T (n = 341) vs Placebo (n=171) Median OS: 25.8 vs 21.7 months](https://image.slidesharecdn.com/36fizazi-110517094520-phpapp01/85/ECCLU-2011-K-Fizazi-Prostate-cancer-drug-development-27-320.jpg)