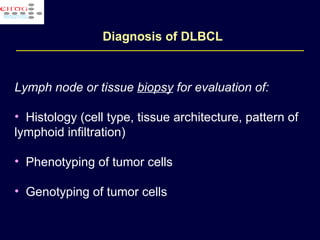
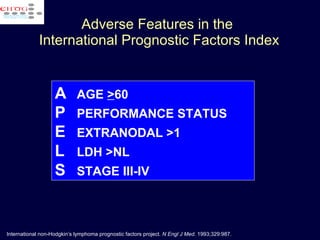
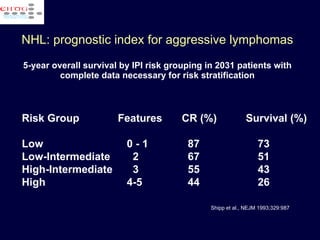
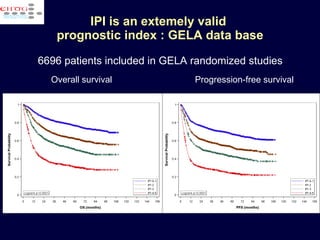
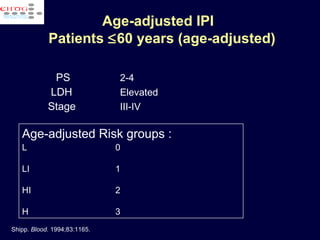
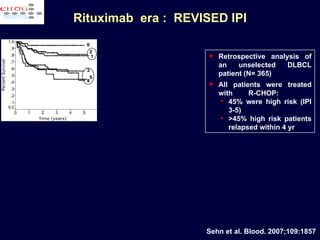
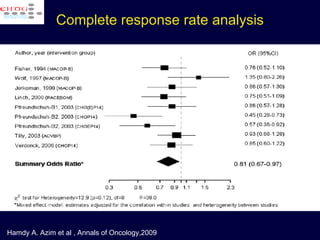
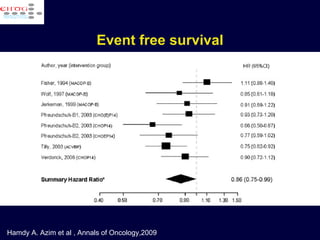
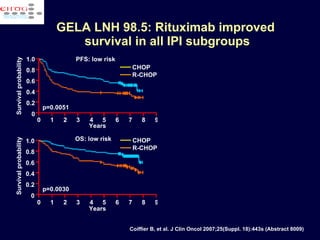
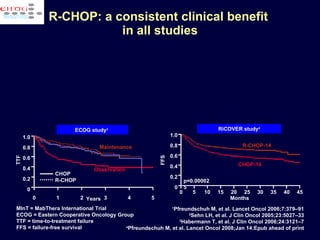
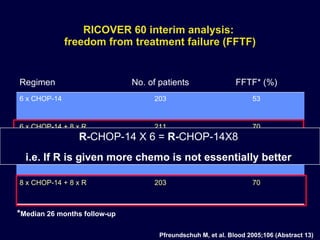
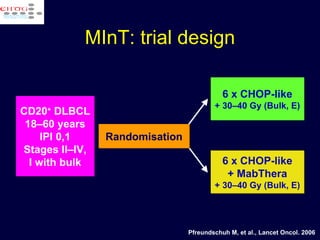
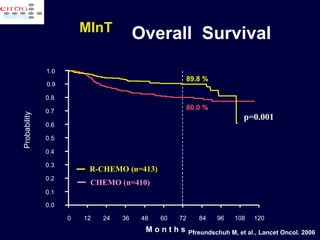
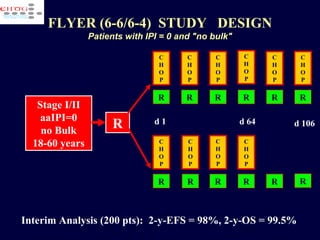
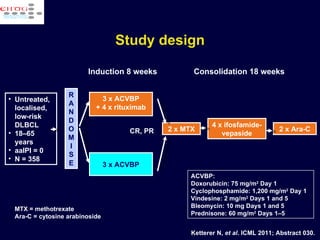
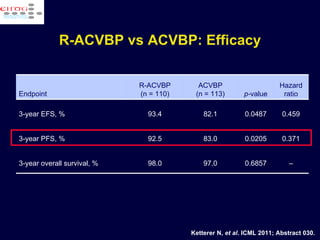
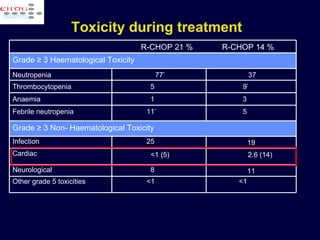
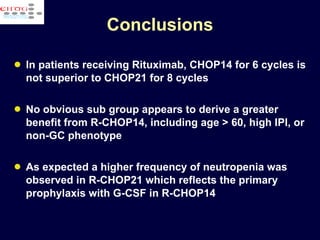
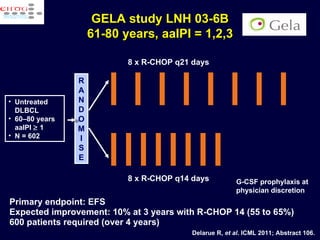
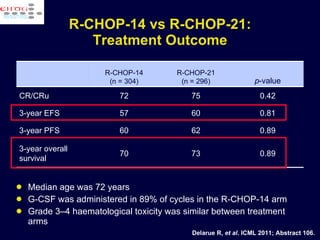
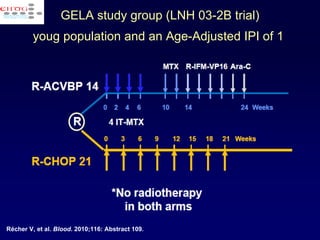
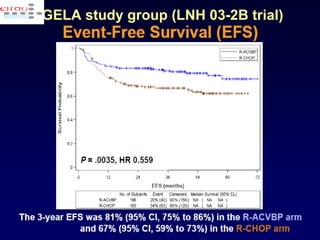
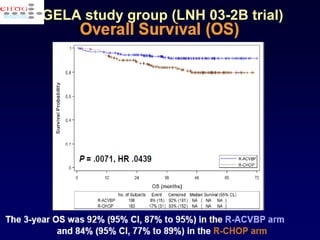
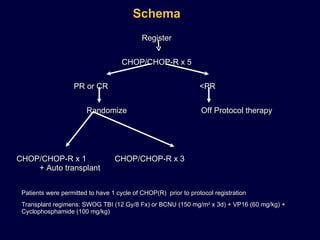
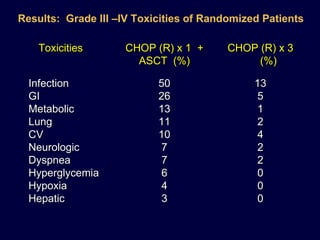
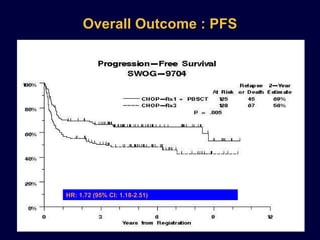
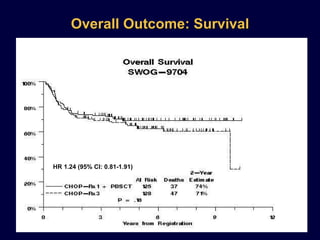
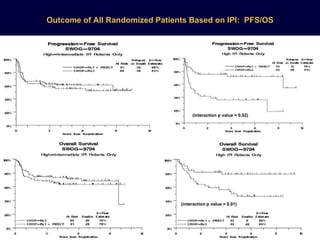
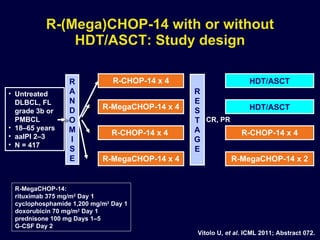
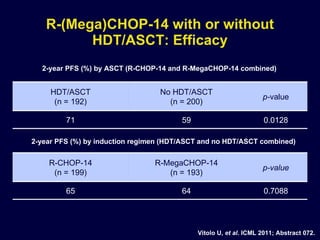
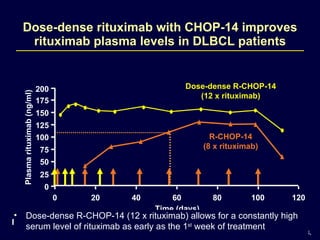
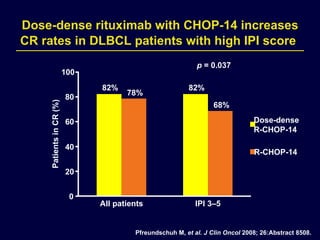
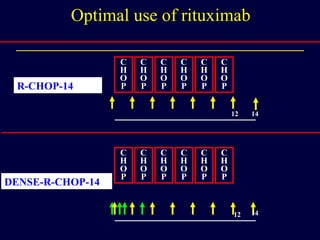
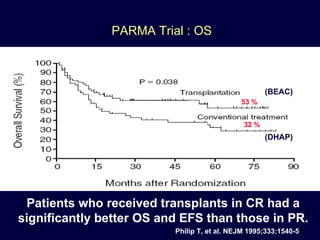
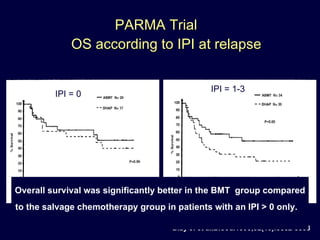
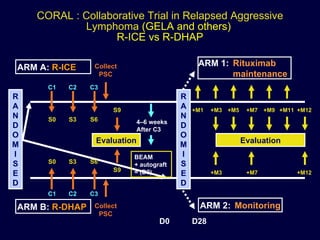
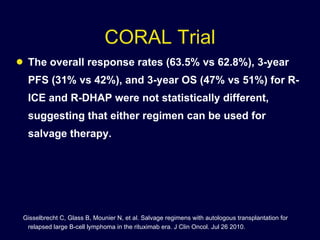
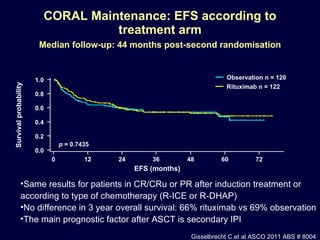
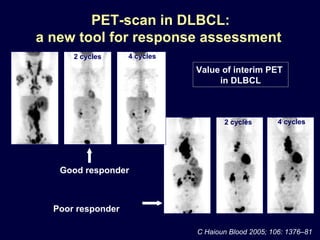
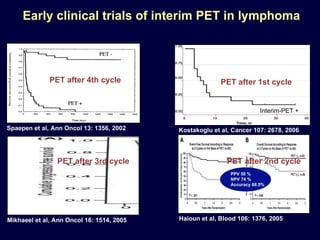
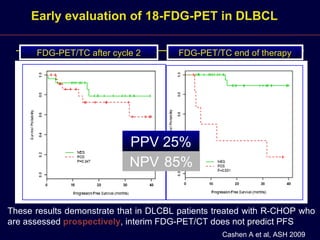
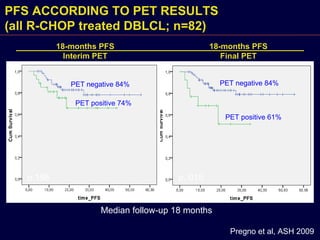
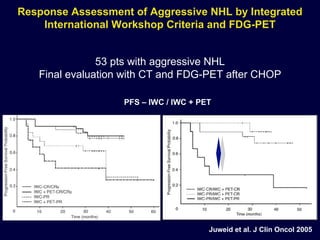
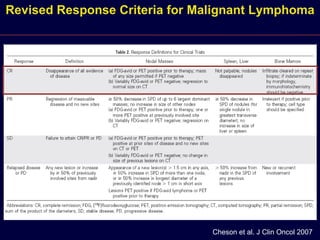
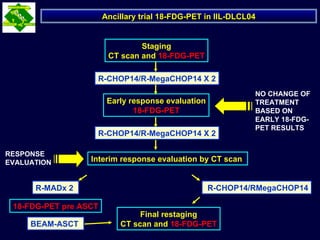
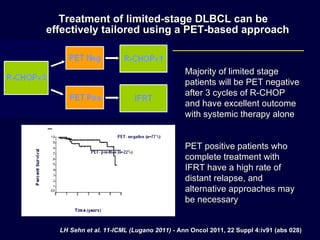
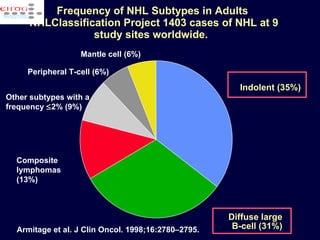
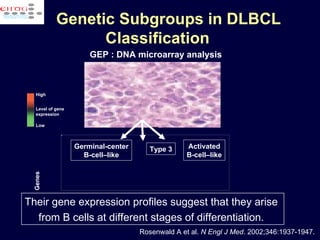
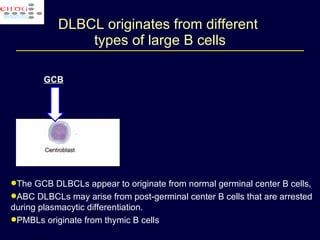
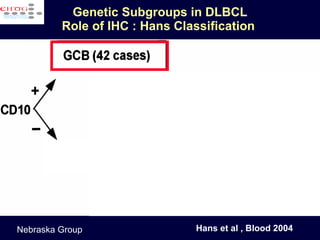
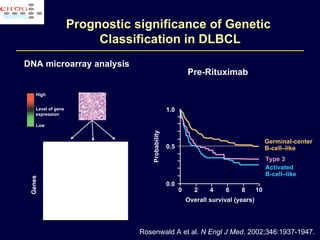
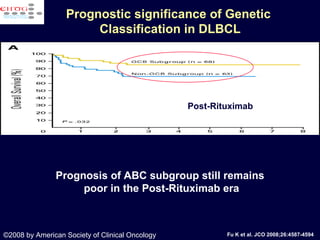
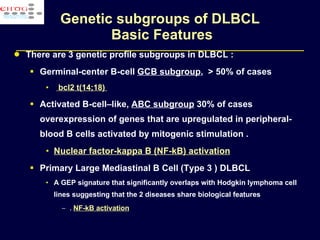
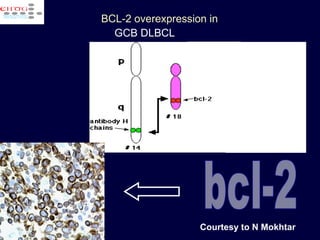
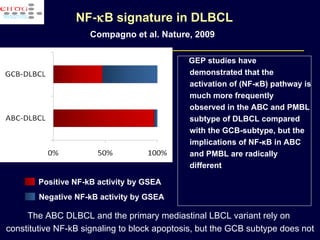
This document discusses treatment of diffuse large B-cell lymphoma (DLBCL). It notes that DLBCL is a heterogeneous disease with genetic subgroups that have different prognoses and responses to treatment. The addition of the antibody rituximab to chemotherapy improves outcomes for DLBCL compared to chemotherapy alone. Strategies discussed to improve outcomes include increasing chemotherapy dose intensity and the potential role of the drug bortezomib for the activated B-cell subtype.










![Efficacy of bortezomib + chemo ( DA-EPOCH) in relapsing DLBCL [ABC-DLBCL vs GCB-DLBCL] Dunleavy et al, Blood 2009; 113:6069 3.4 months 10.8 months P = .003 improved response rates for ABC compared with GCB. (83% ORR with 41,5% CR vs. 13% %; P < .001 )](https://image.slidesharecdn.com/1azim-111130100148-phpapp02/85/1-azim-23-320.jpg)



![CNS Disease in Aggressive Lymphomas Risk factors for CNS disease identified from phase III study (N = 444) in elderly, untreated patients with aggressive lymphomas [1] Most significant risk factors Special sites : Testicle epidura, sinus, orbit Clinical stage IPI score Independent risk factors for CNS disease recurrence [2] Elevated LDH > 1 extranodal disease site 1. Bj örkholm M, et al. Ann Oncol. 2007;18:1085-1089. 2. Boehme V, et al. Ann Oncol. 2007;18:149-157. Risk Factor CNS Disease P Value No Yes Testis involvement No 210 15 .002 Yes 4 4 Clinical stage II 145 4 .005 III 113 6 IV 156 19 IPI score 1 114 7 .035 2 145 15 3 86 4](https://image.slidesharecdn.com/1azim-111130100148-phpapp02/85/1-azim-27-320.jpg)