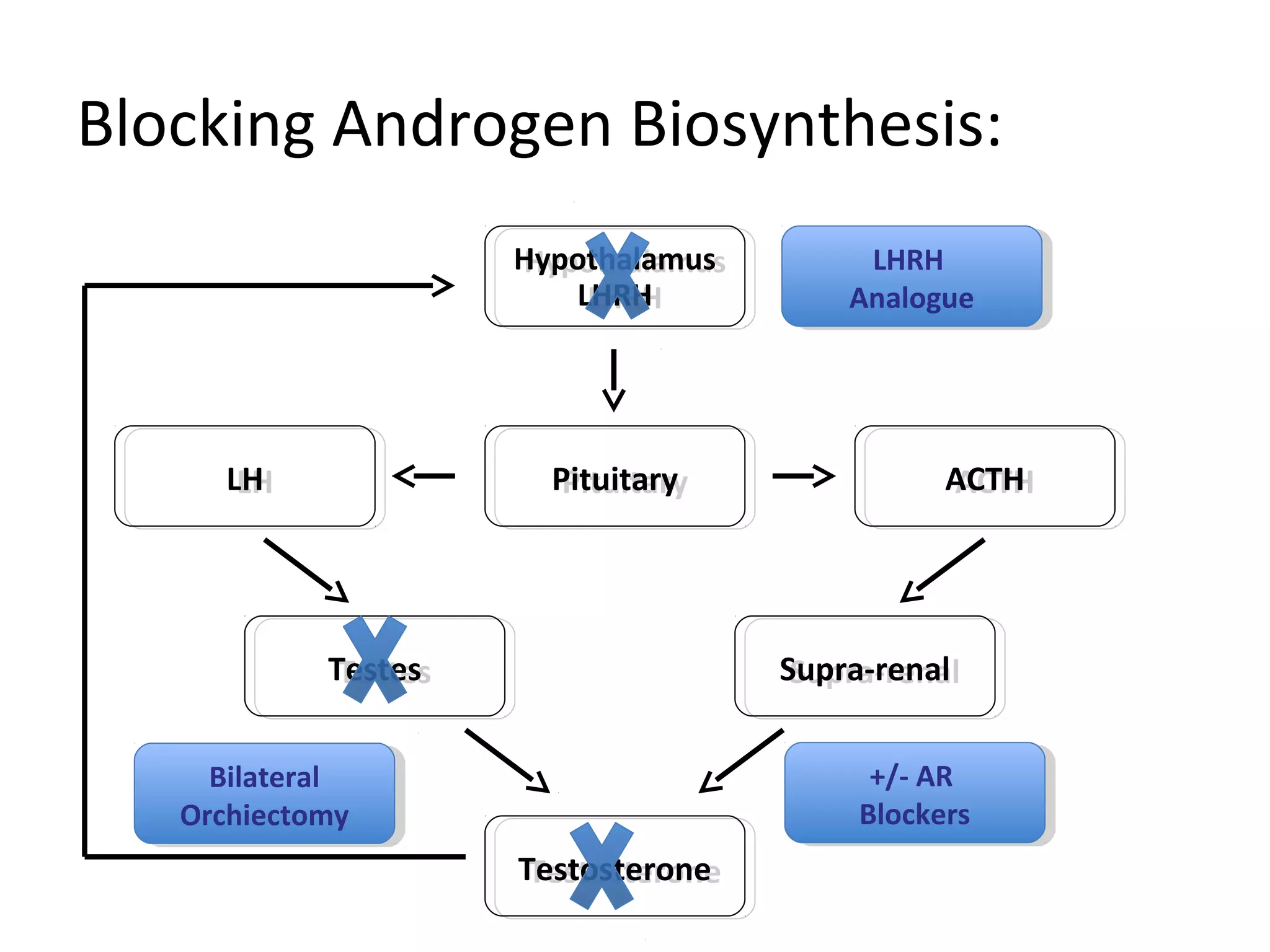
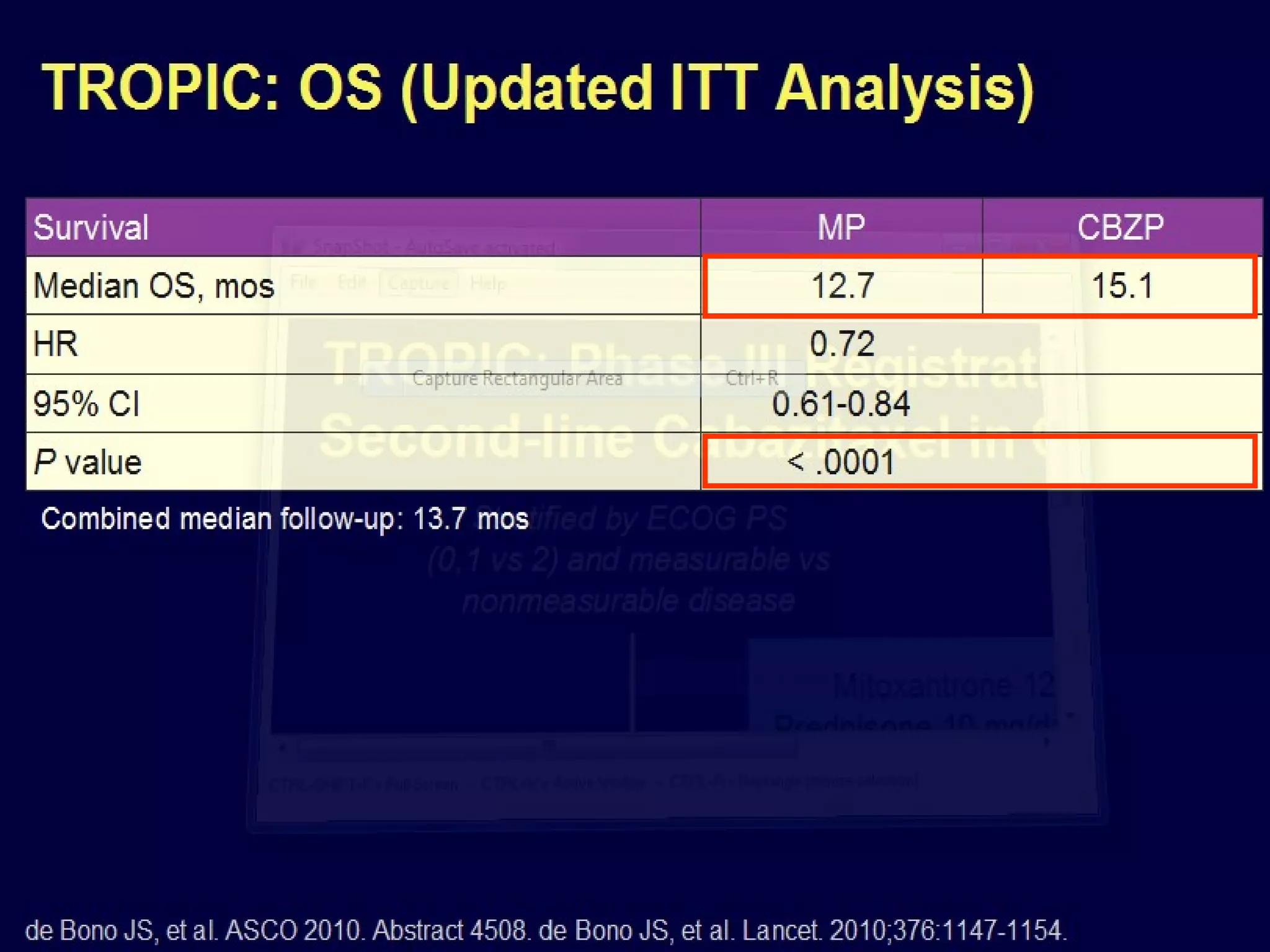
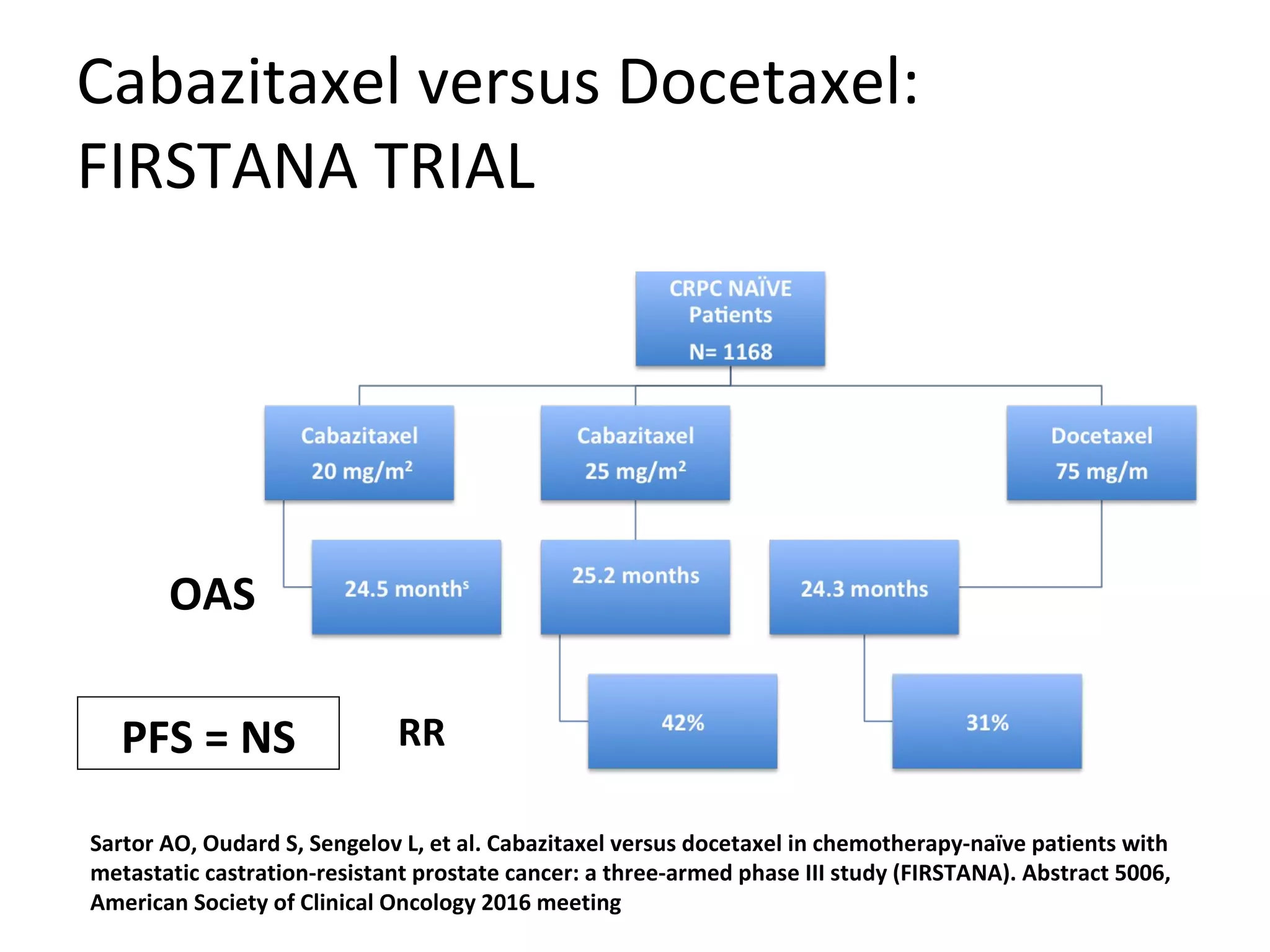
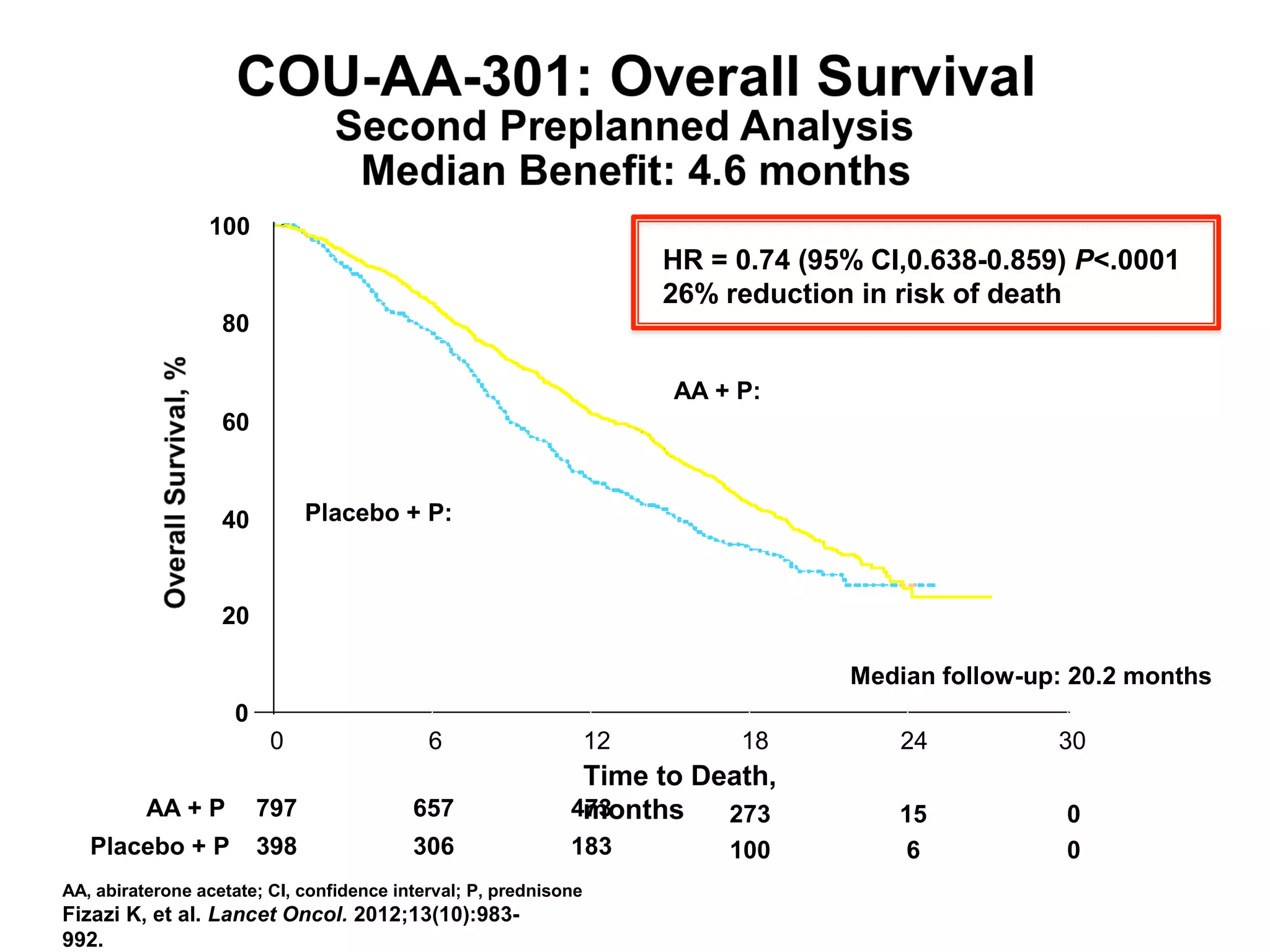
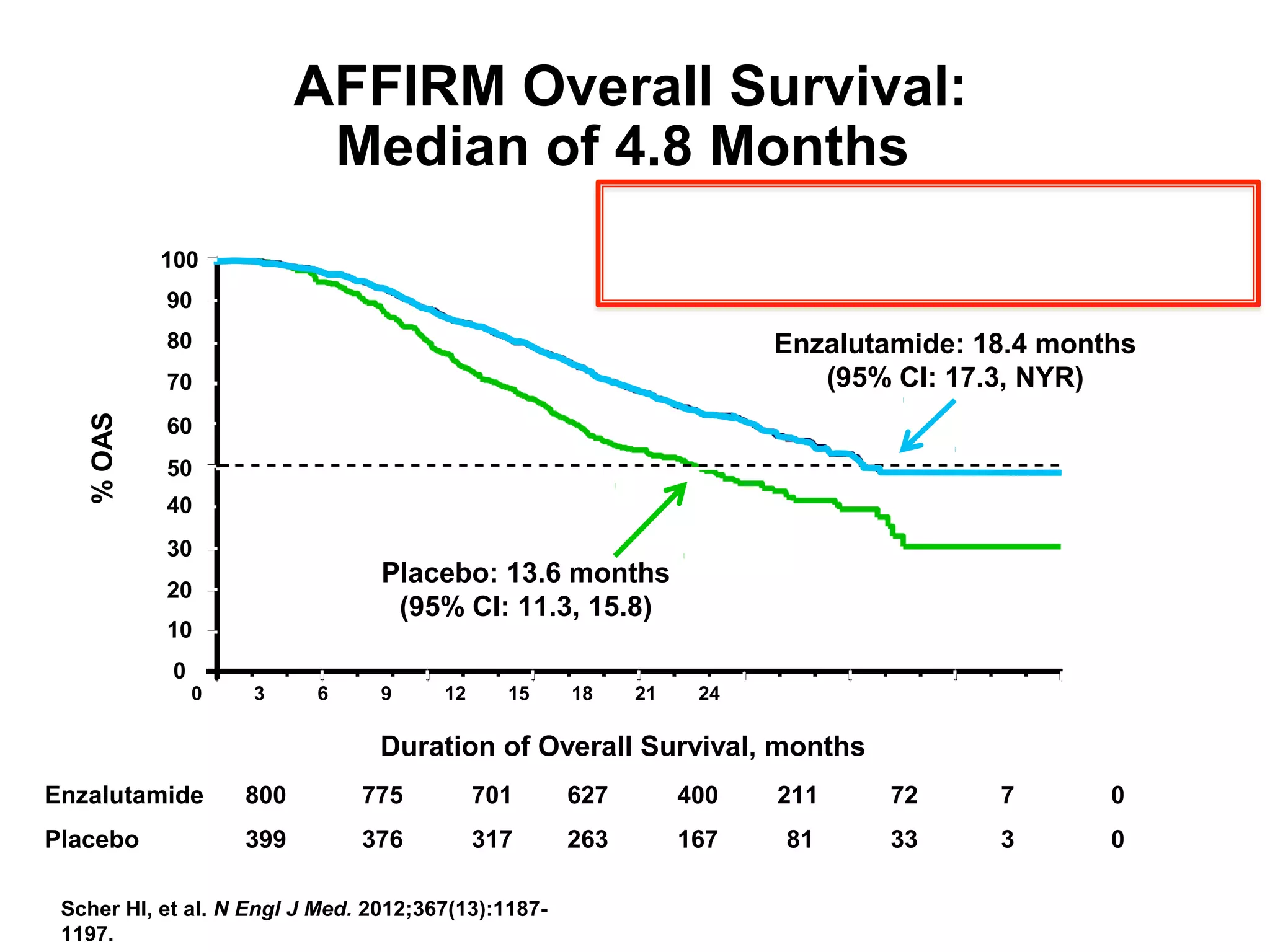
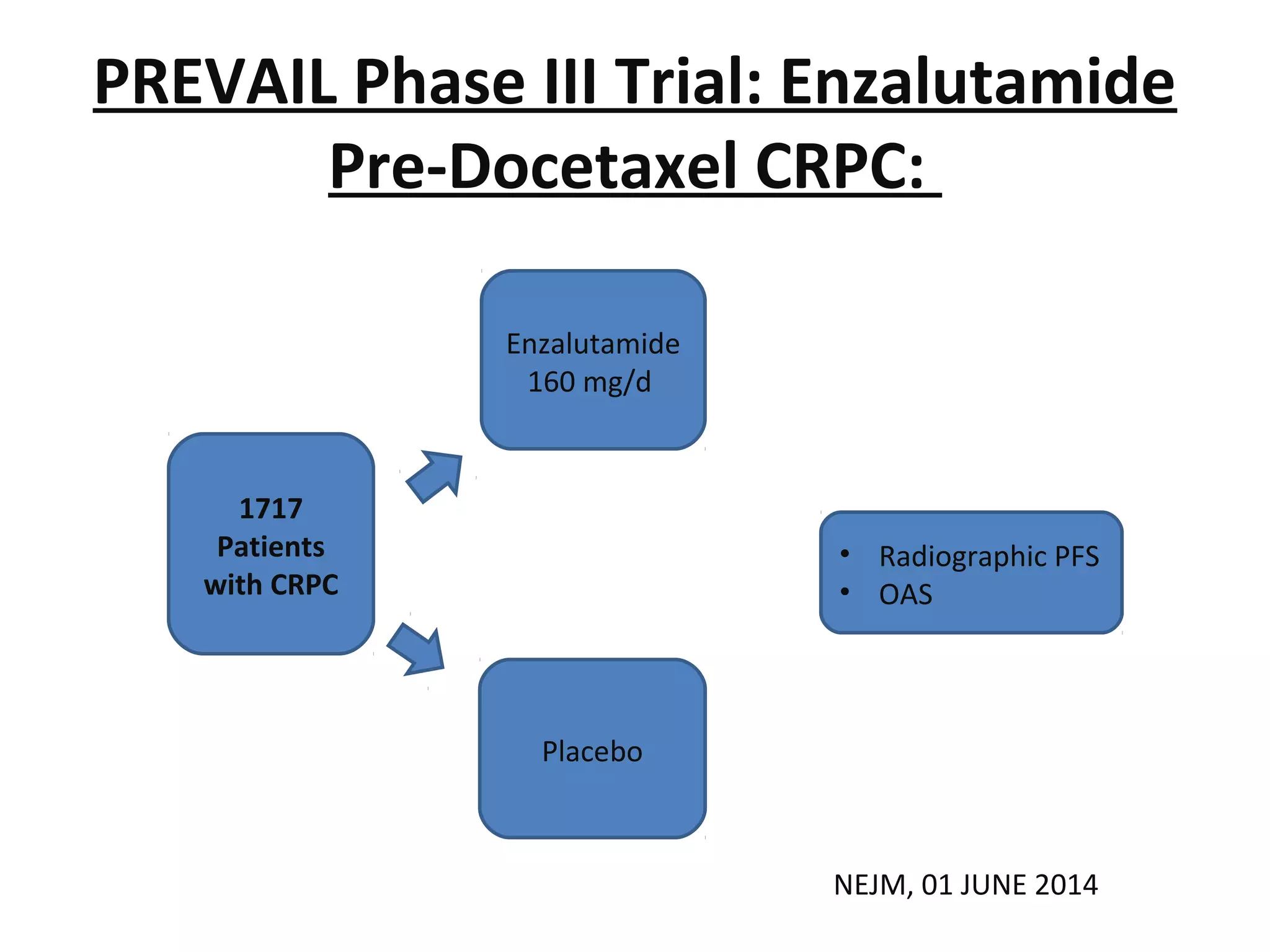
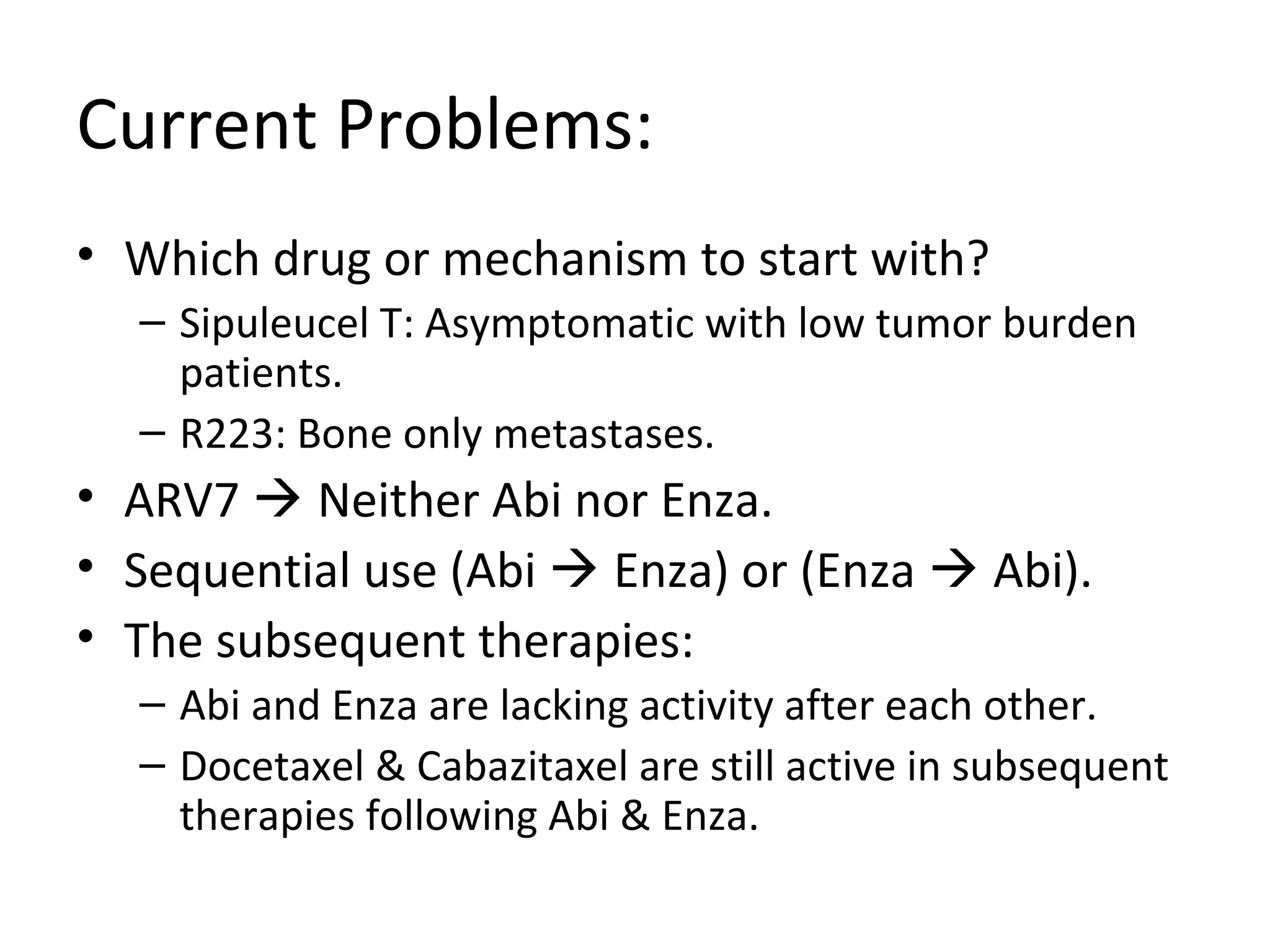
The document discusses the management of castration-resistant prostate cancer (CRPC), highlighting various therapeutic strategies, including androgen deprivation therapy (ADT), androgen receptor blockers, and cytotoxic chemotherapy. It details the efficacy of drugs such as abiraterone acetate and enzalutamide, supported by results from clinical trials, emphasizing their roles in improving overall survival and risk of disease progression. The author notes ongoing challenges in treatment selection and the necessity for proper patient assessment to optimize outcomes.