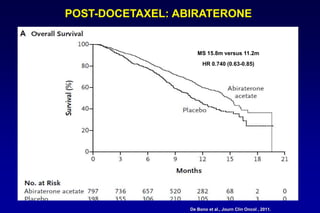
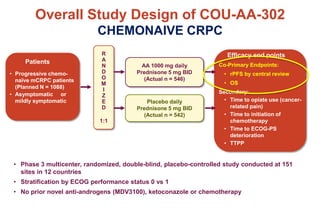
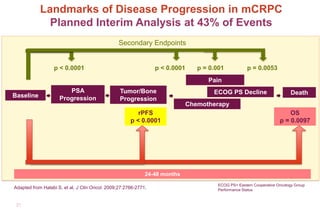
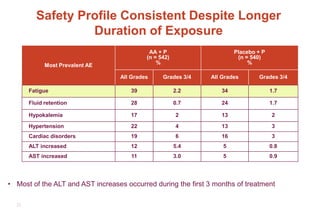
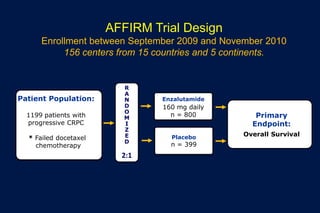
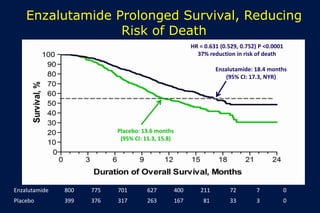
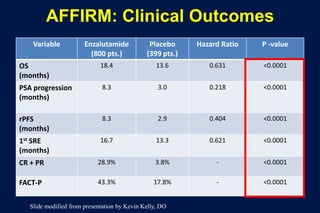
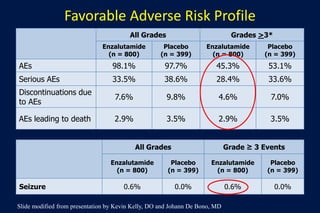
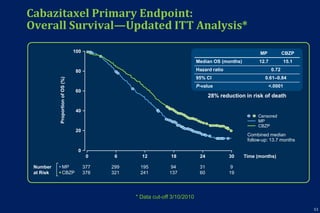
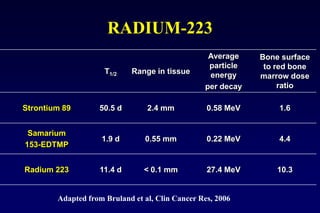
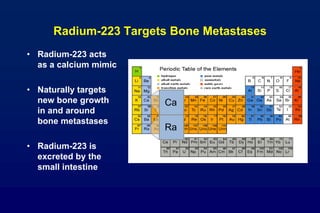
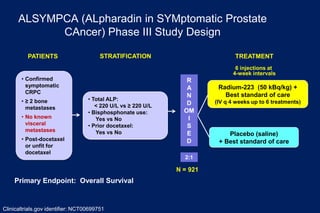
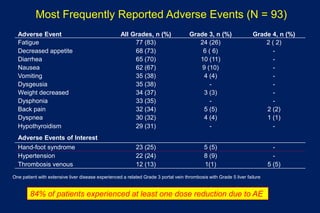
This document summarizes key information about prostate cancer including:
1. Risk factors such as age, ethnicity, family history, and diet.
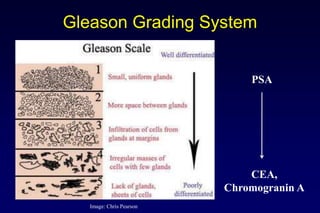
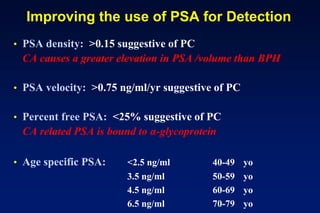
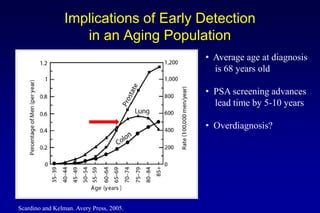
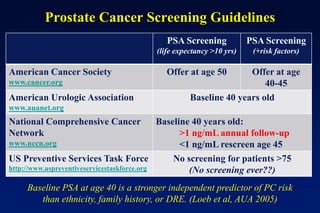
2. Diagnostic tools like PSA testing which is imperfect but can be improved through measures of density and velocity.
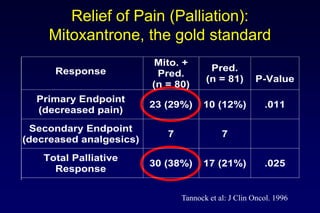
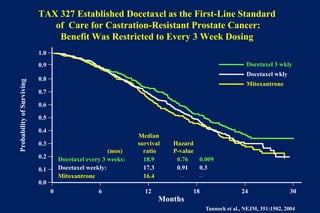
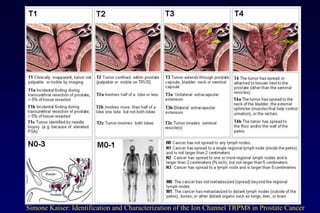
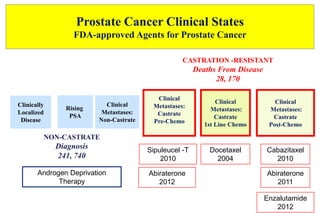
3. Clinical stages from localized to metastatic disease and treatments available at each stage including androgen deprivation therapy and newer agents.
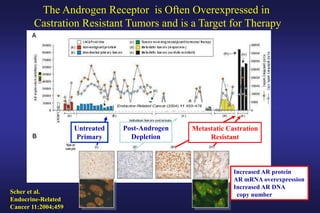
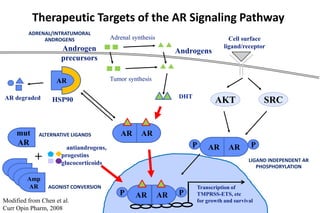
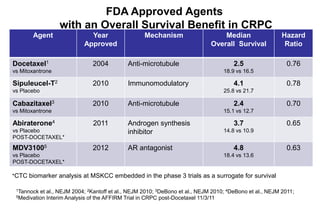
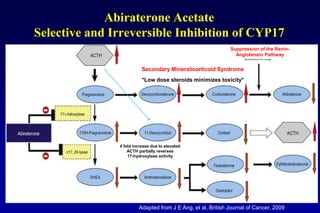
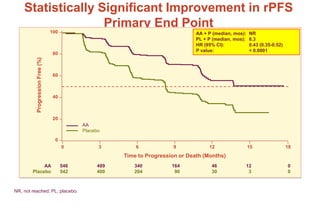
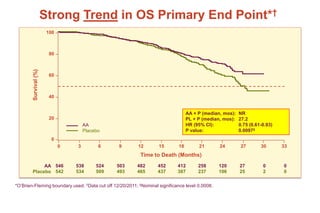
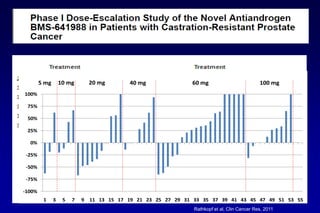
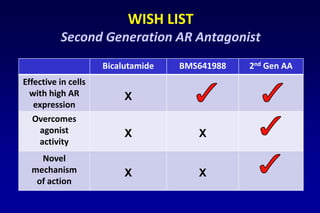
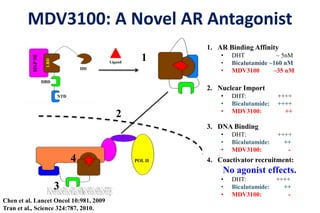
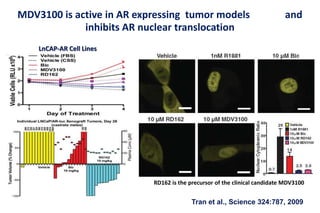
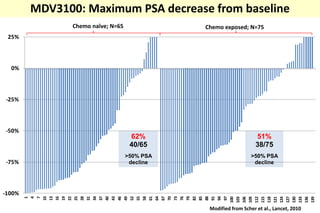
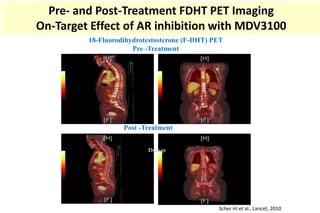
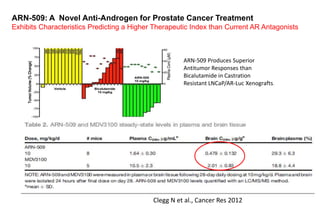
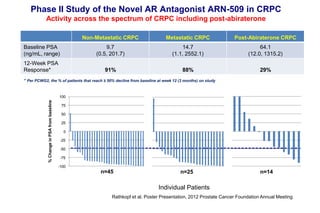
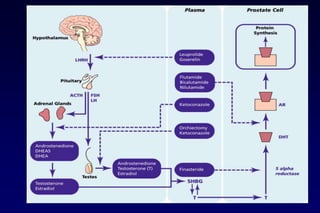
4. Ongoing research into better targeting the androgen receptor pathway which remains important even in advanced "castrate resistant" prostate cancer.

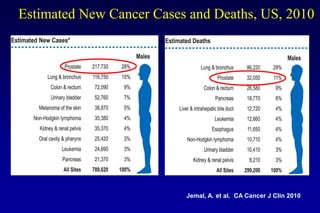
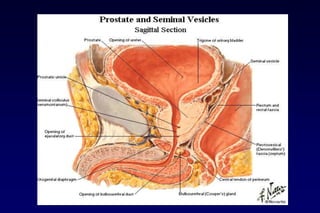
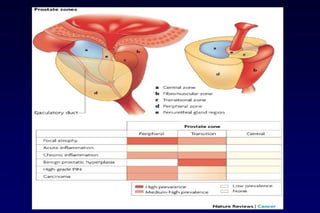





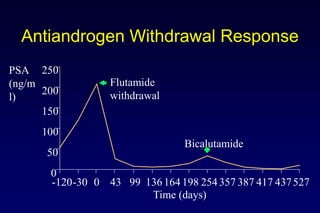
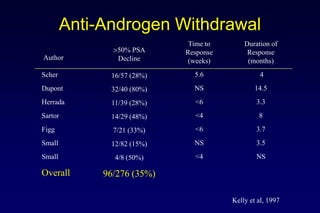

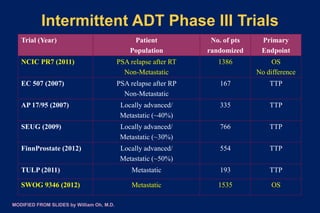
![[TITLE]
Overall survival with Intermittent Hormonal Therapy is
NOT “NONINFERIOR” to Continuous Therapy
(Translation: it is POTENTIALLY inferior)
Modified Slide](https://image.slidesharecdn.com/prostate101housestaffwebversion2013-130513152630-phpapp02/85/Prostate-101-22-320.jpg)