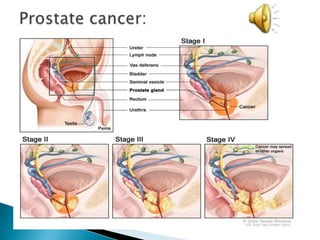
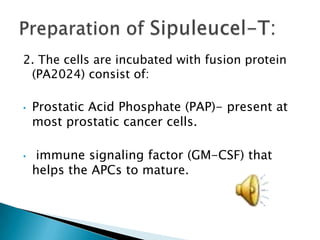
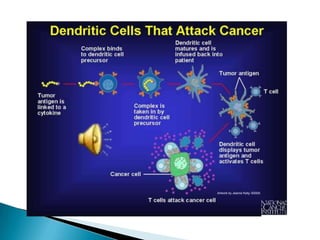
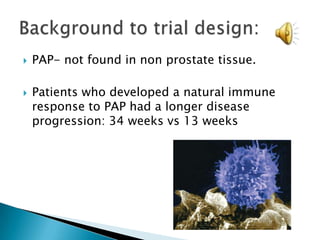
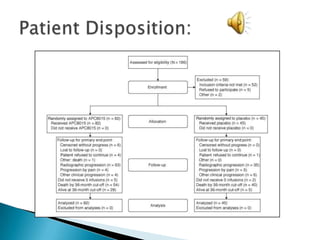
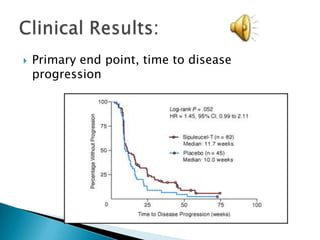
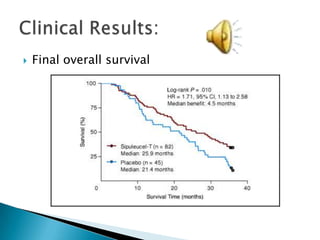
This Phase III clinical trial evaluated the efficacy and safety of Sipuleucel-T immunotherapy compared to placebo for asymptomatic metastatic hormone refractory prostate cancer patients. 127 patients were randomly assigned to receive three infusions of either Sipuleucel-T or placebo every two weeks. The primary endpoints were time to disease progression and overall survival. Results showed that median time to disease progression was 11.7 weeks for Sipuleucel-T versus 10 weeks for placebo, and median overall survival was 25.9 months for Sipuleucel-T versus 21.4 months for placebo. No patients discontinued the trial due to treatment toxicity. The study suggests Sipuleucel-T may provide a survival advantage