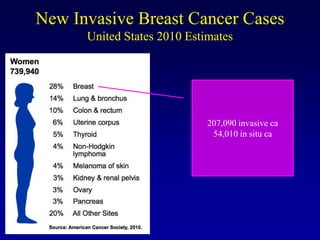
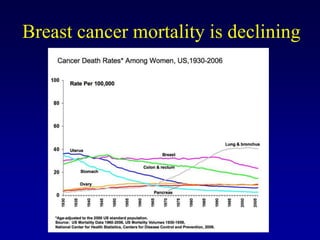
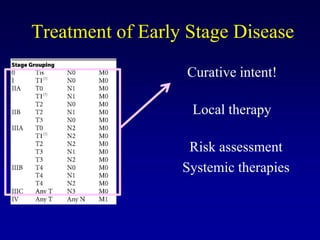
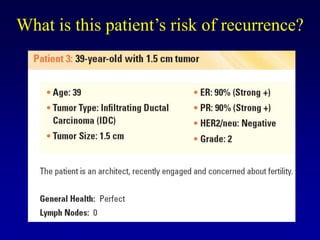
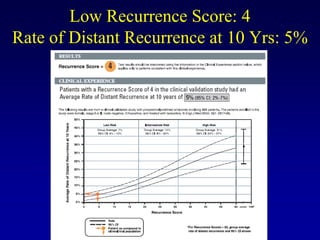
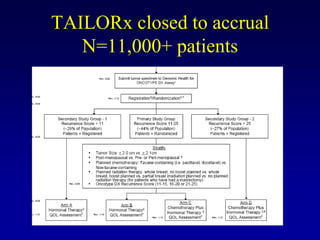
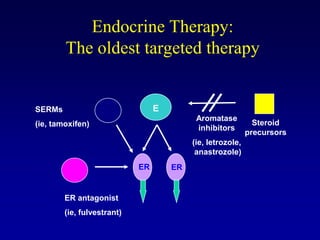
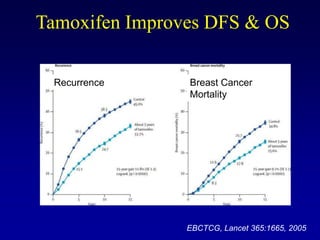
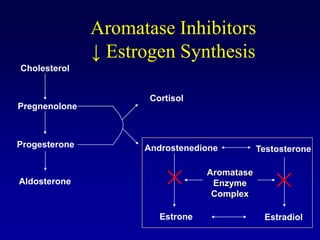
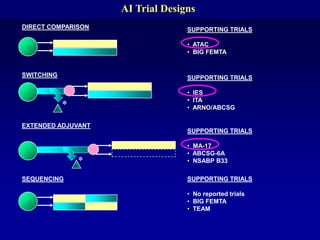
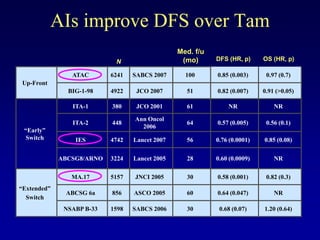
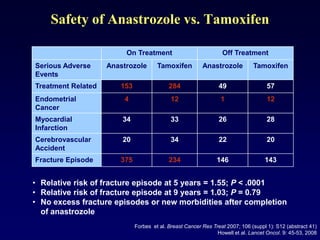
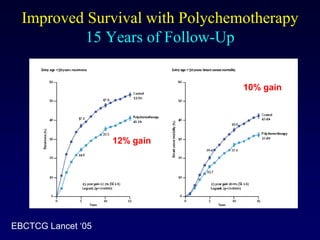
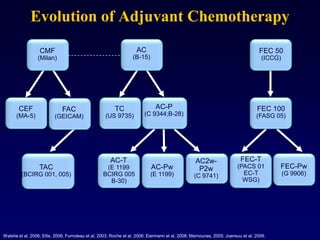
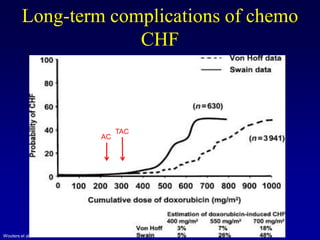
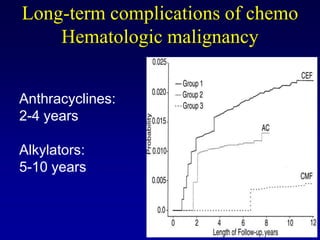
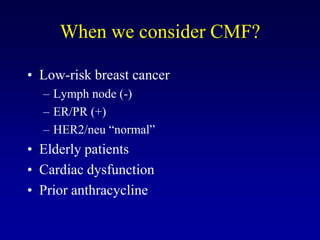
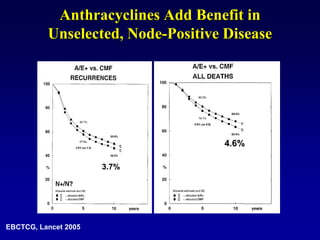
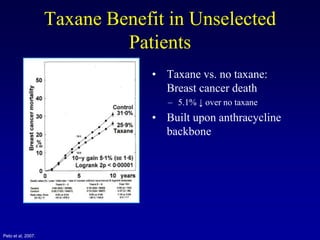
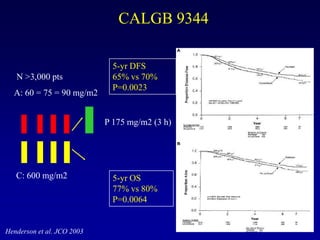
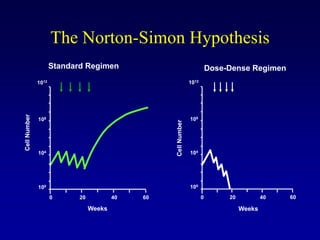
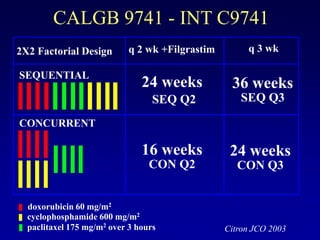
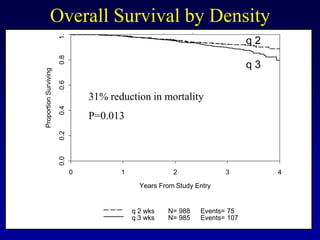
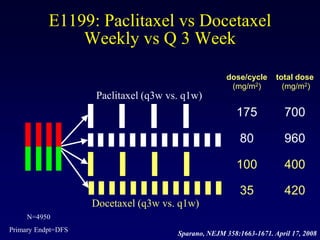
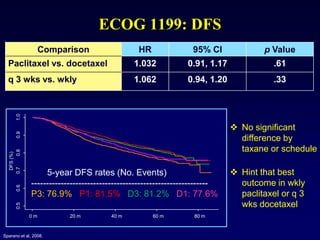
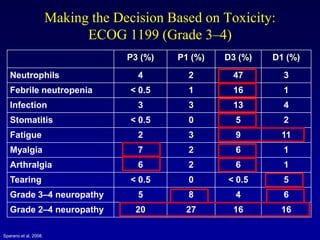
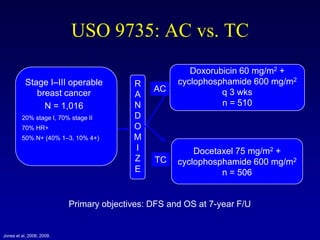
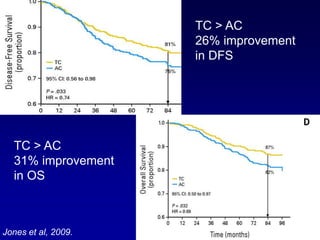
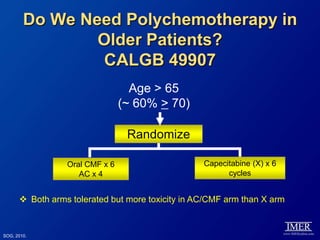
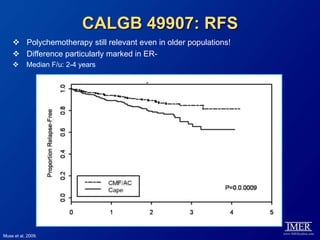
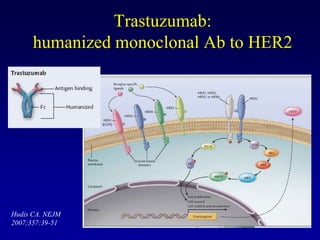
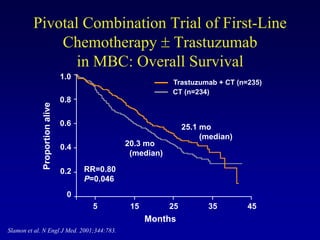
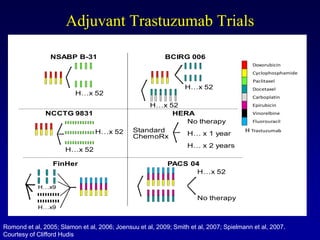
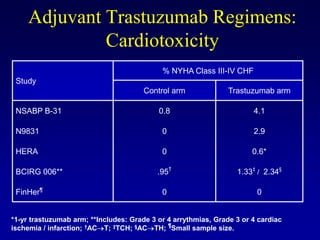
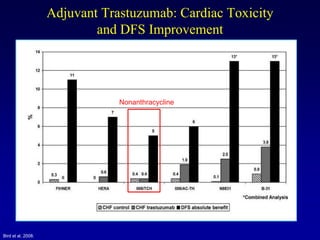
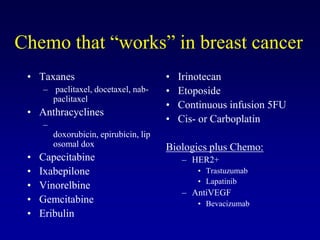
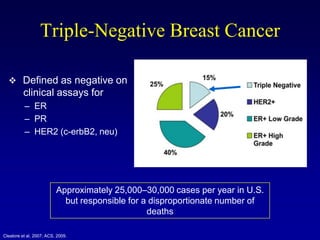
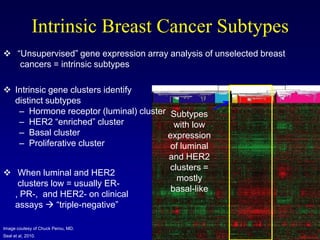
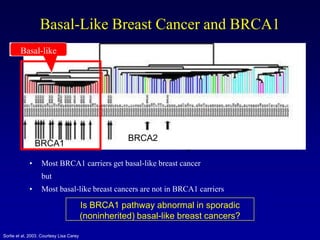
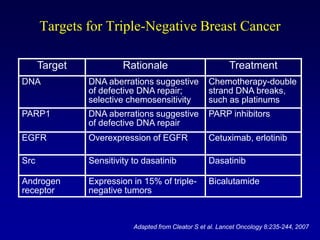
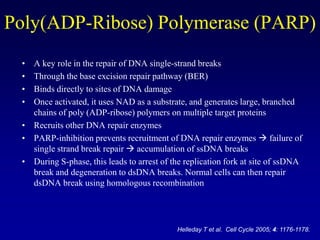
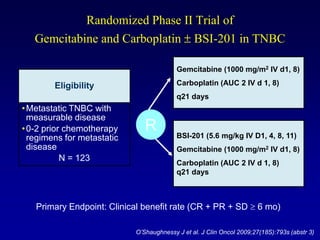
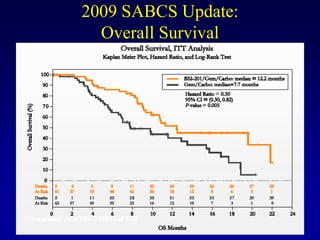
This document provides an overview of breast cancer therapy. It discusses trends in breast cancer incidence in the United States, declining mortality rates, and treatment approaches for early stage disease including local and systemic therapies guided by risk assessment. Key aspects of risk assessment using tools like Oncotype DX are outlined. The roles of endocrine therapy and chemotherapy in adjuvant treatment are summarized, including evolving regimens and trial results demonstrating improved outcomes. Potential toxicities of different systemic therapies are also highlighted.