1. Resins are secondary plant metabolites that are insoluble in water but soluble in organic solvents like alcohol. They are classified based on their formation, chemical nature, and occurrence with other compounds. Methods to extract resins include solvent extraction and steam distillation.
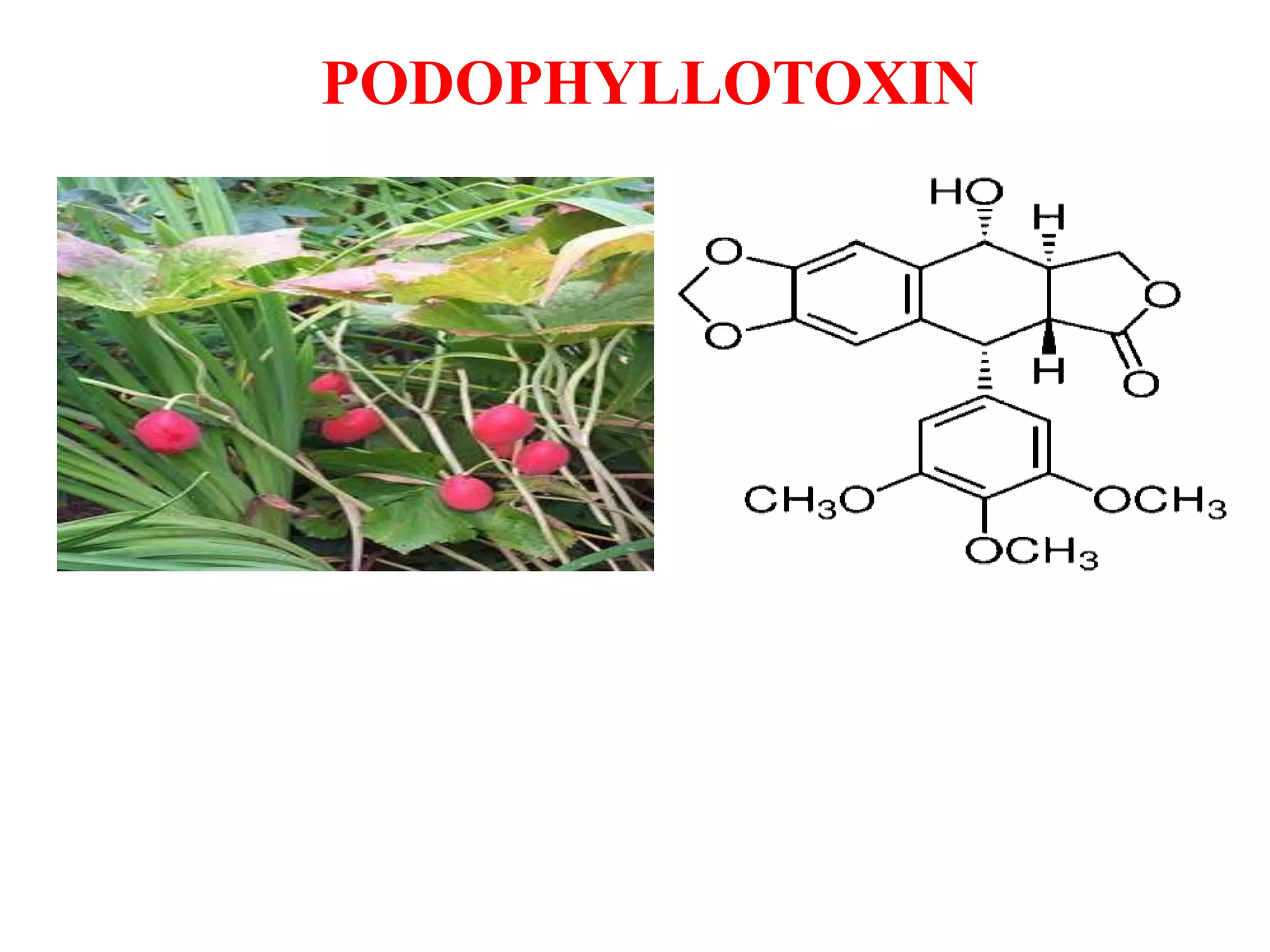
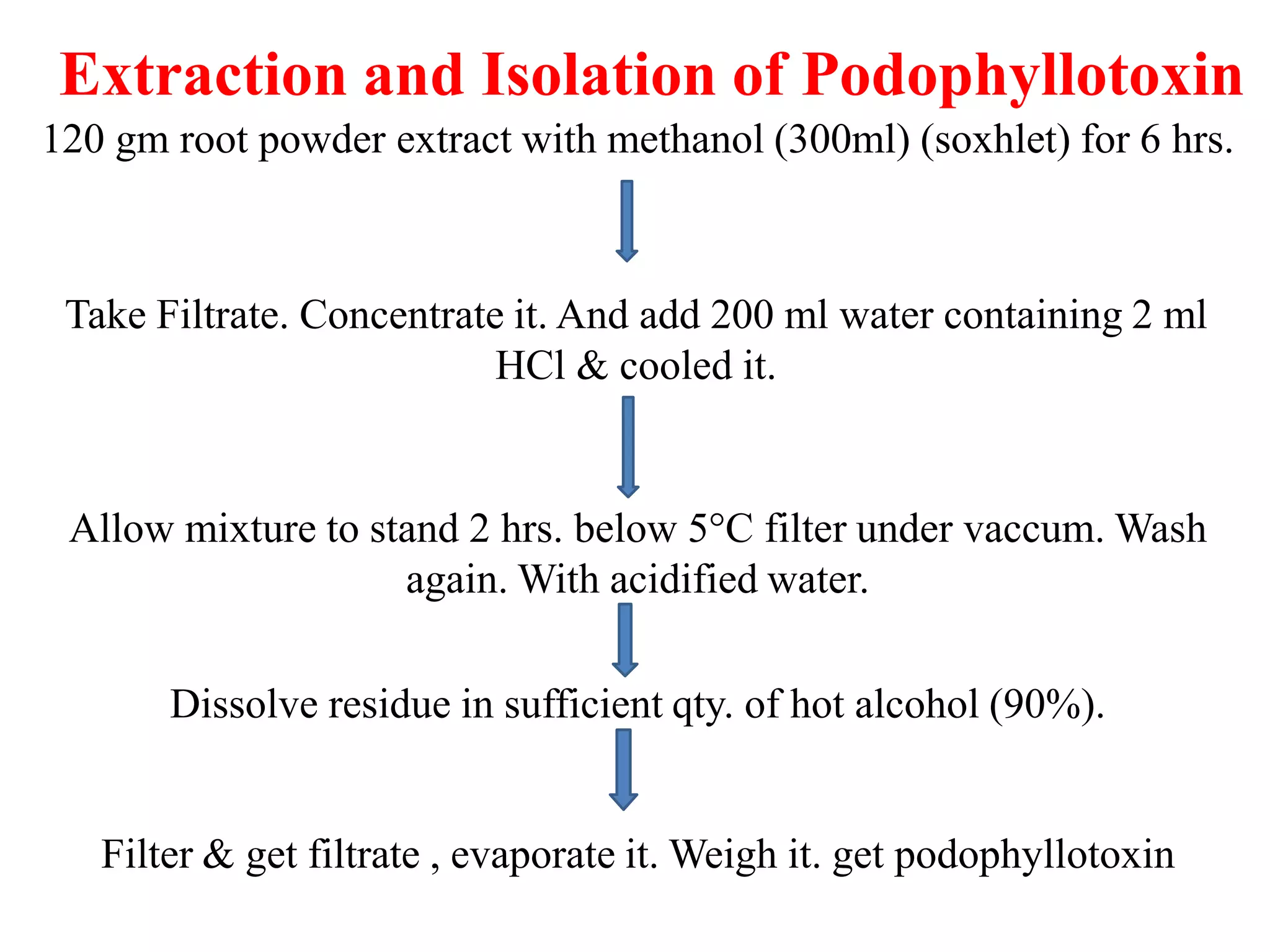
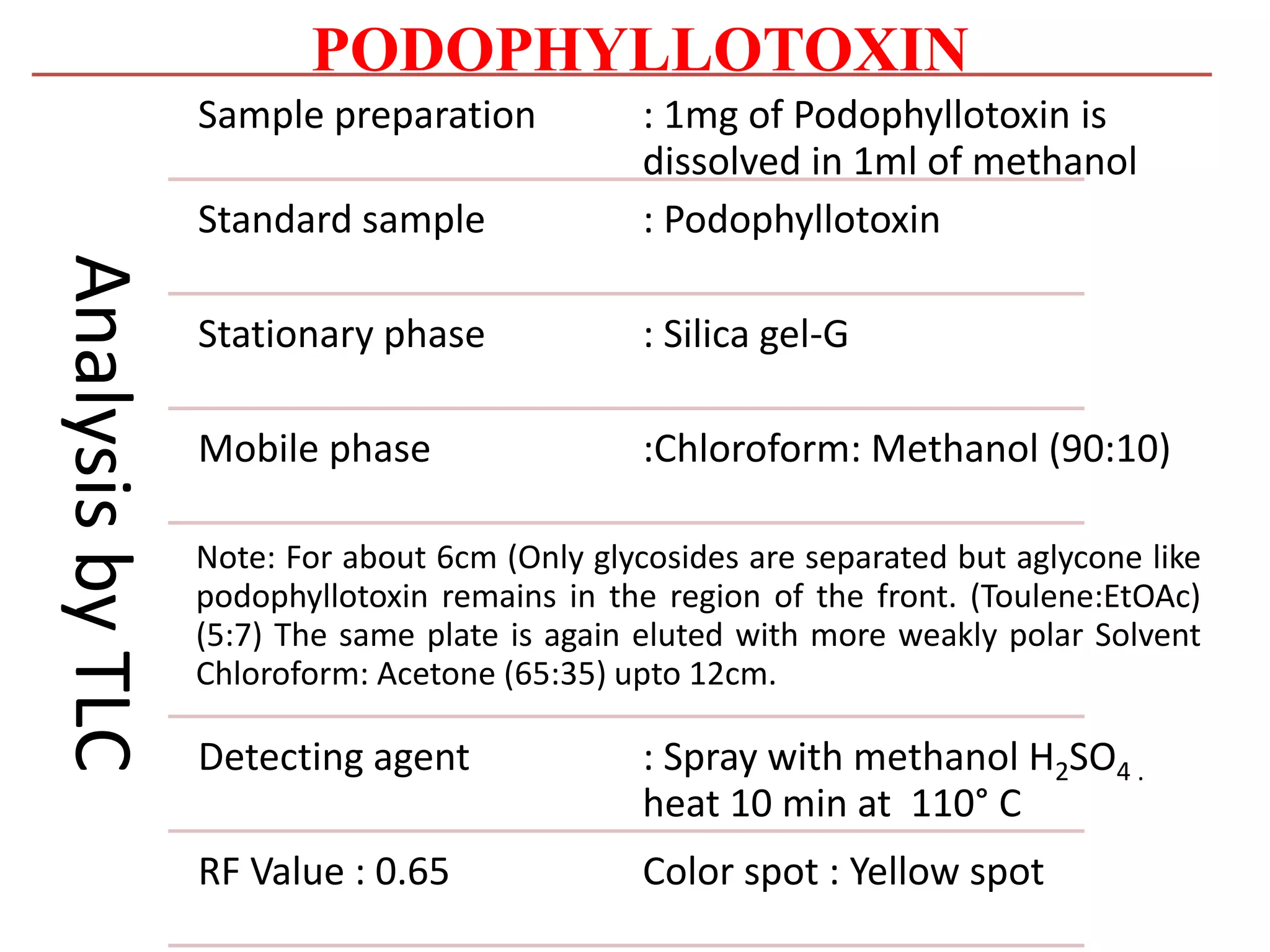
2. Podophyllotoxin is extracted from the roots and rhizomes of Podophyllum species. It has anticancer properties and is used to treat genital warts. It is isolated from plant material via solvent extraction and crystallization. Analysis is done using TLC and HPLC.
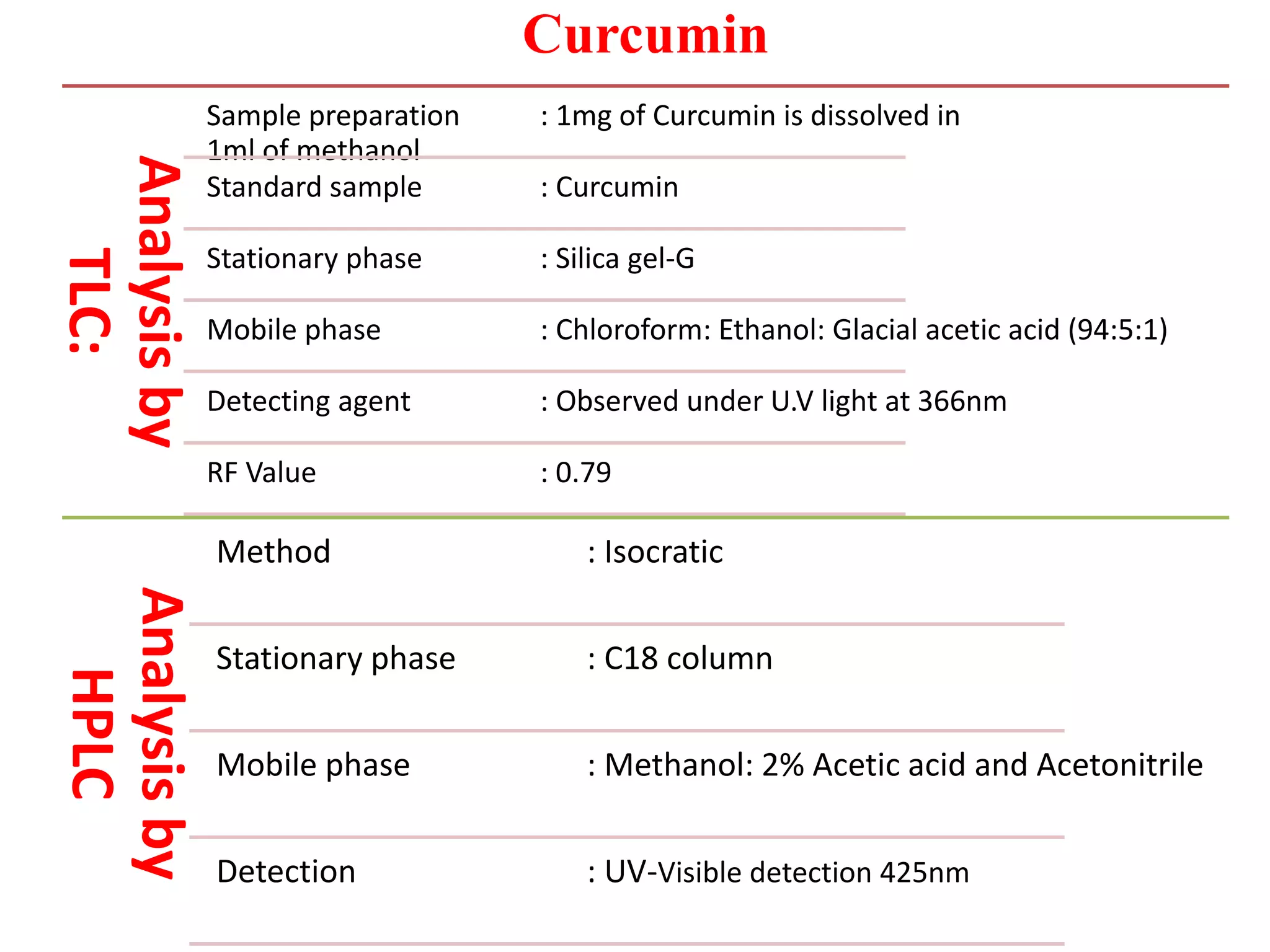
3. Curcumin is the main active compound providing the yellow color of turmeric. It is isolated from turmeric rhizomes by solvent extraction and crystall