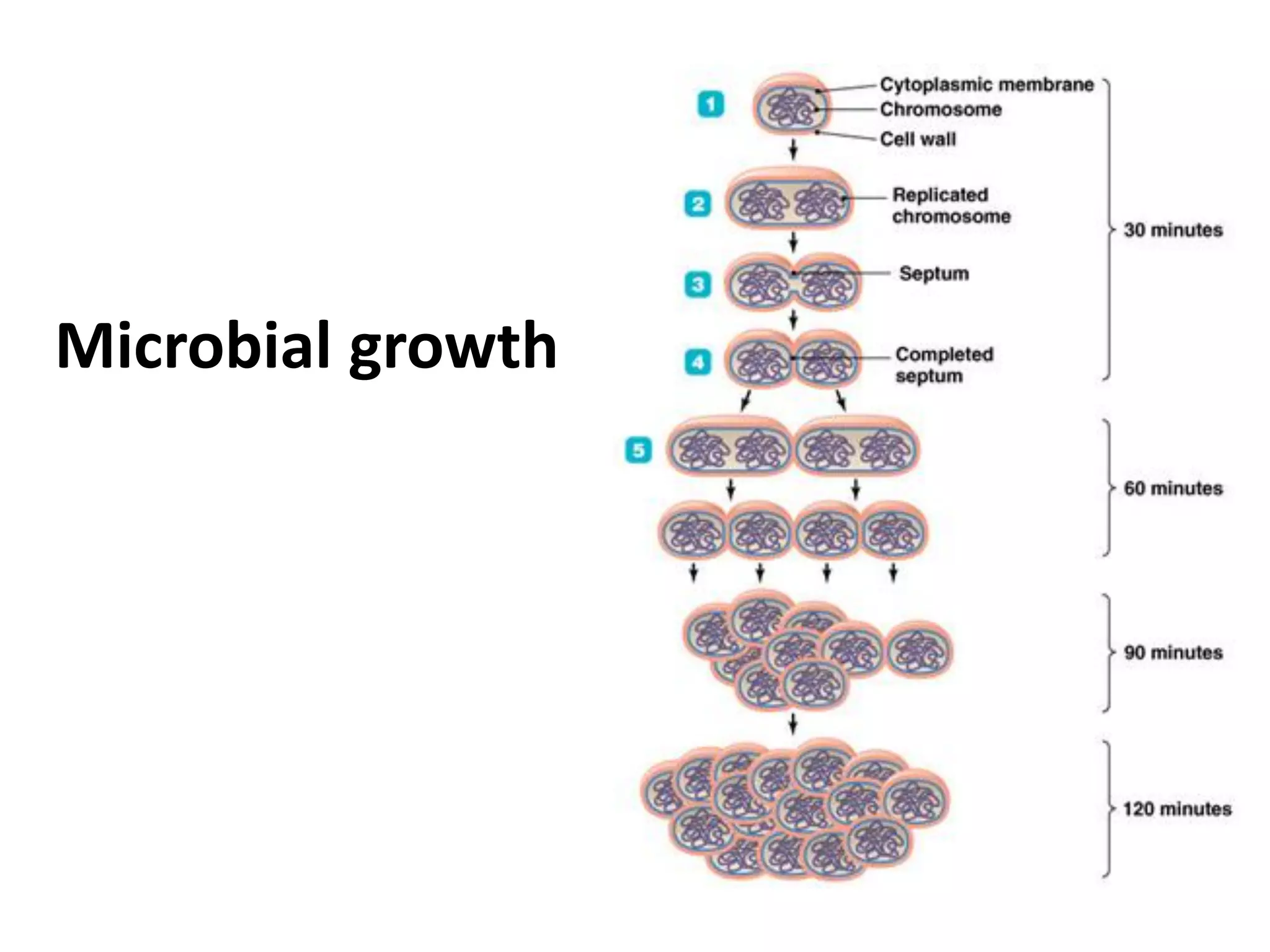
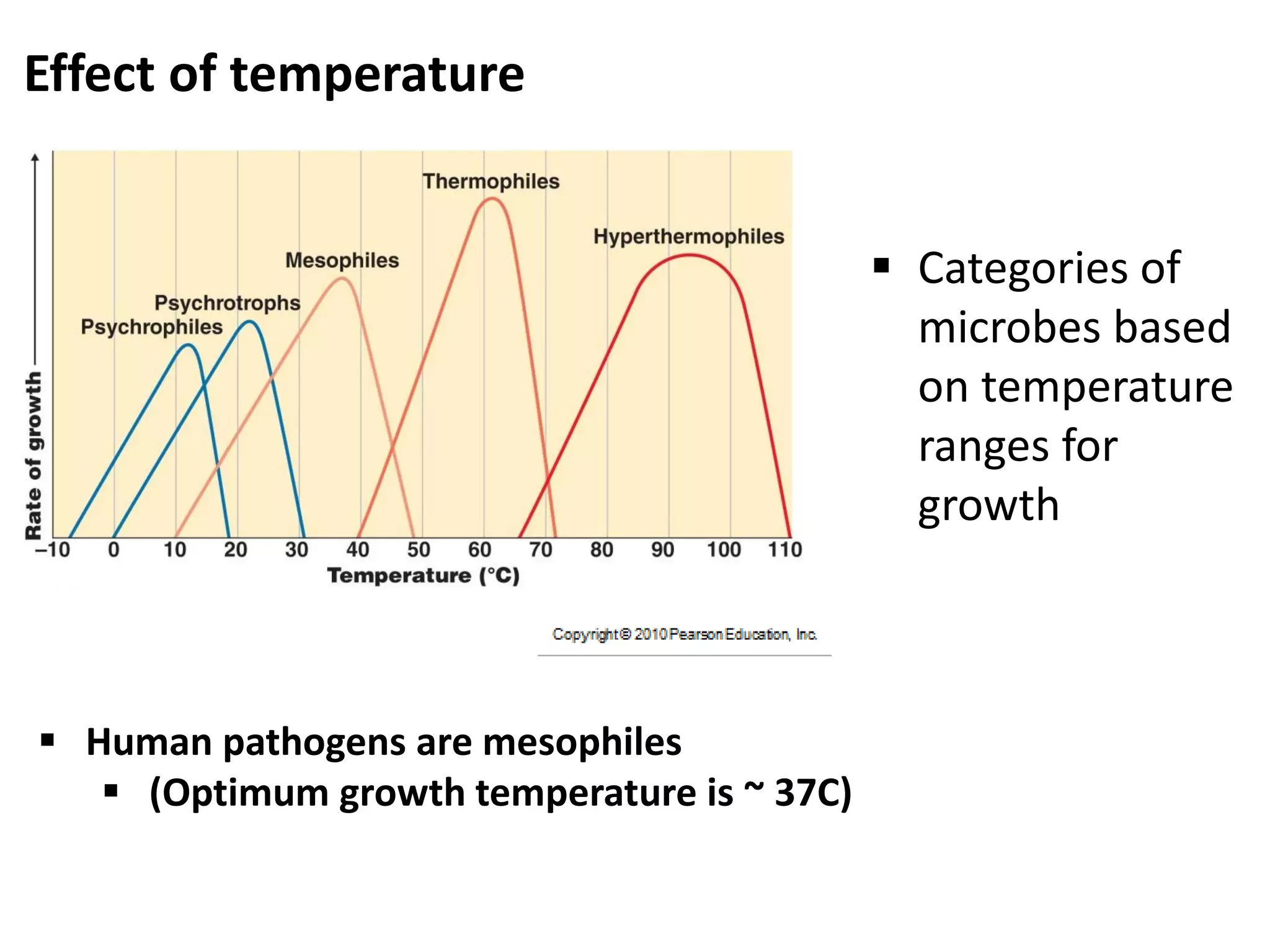
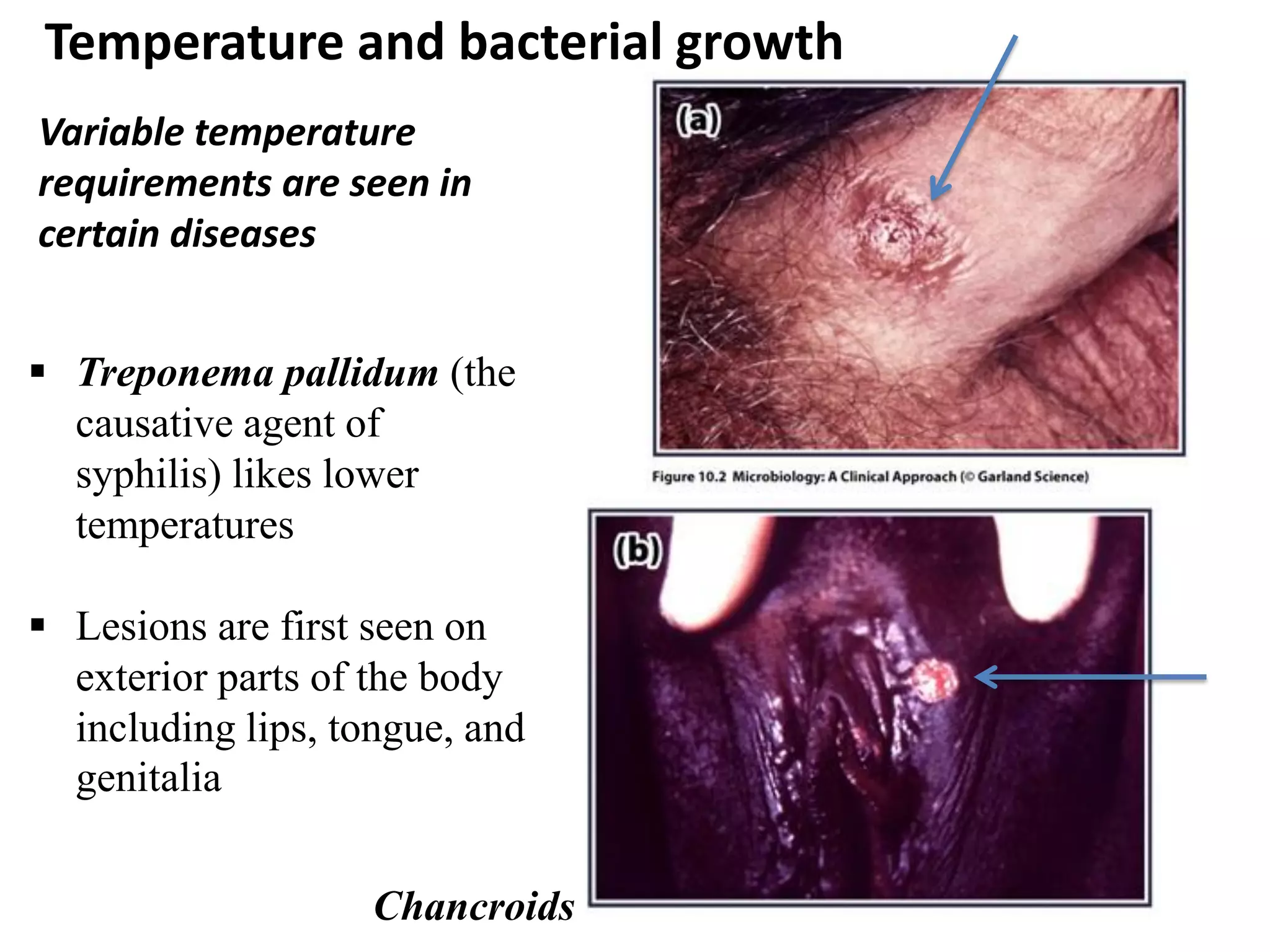
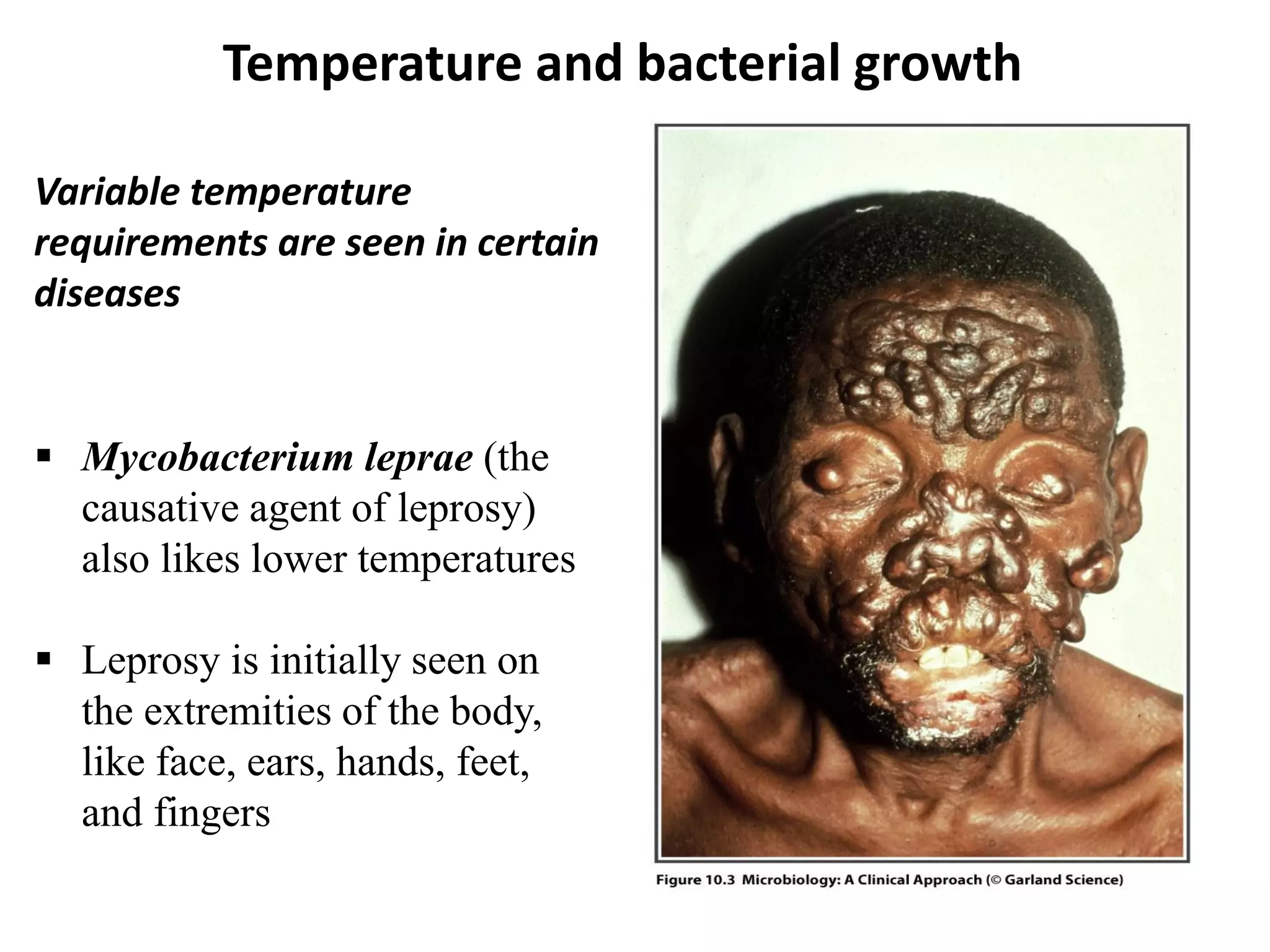
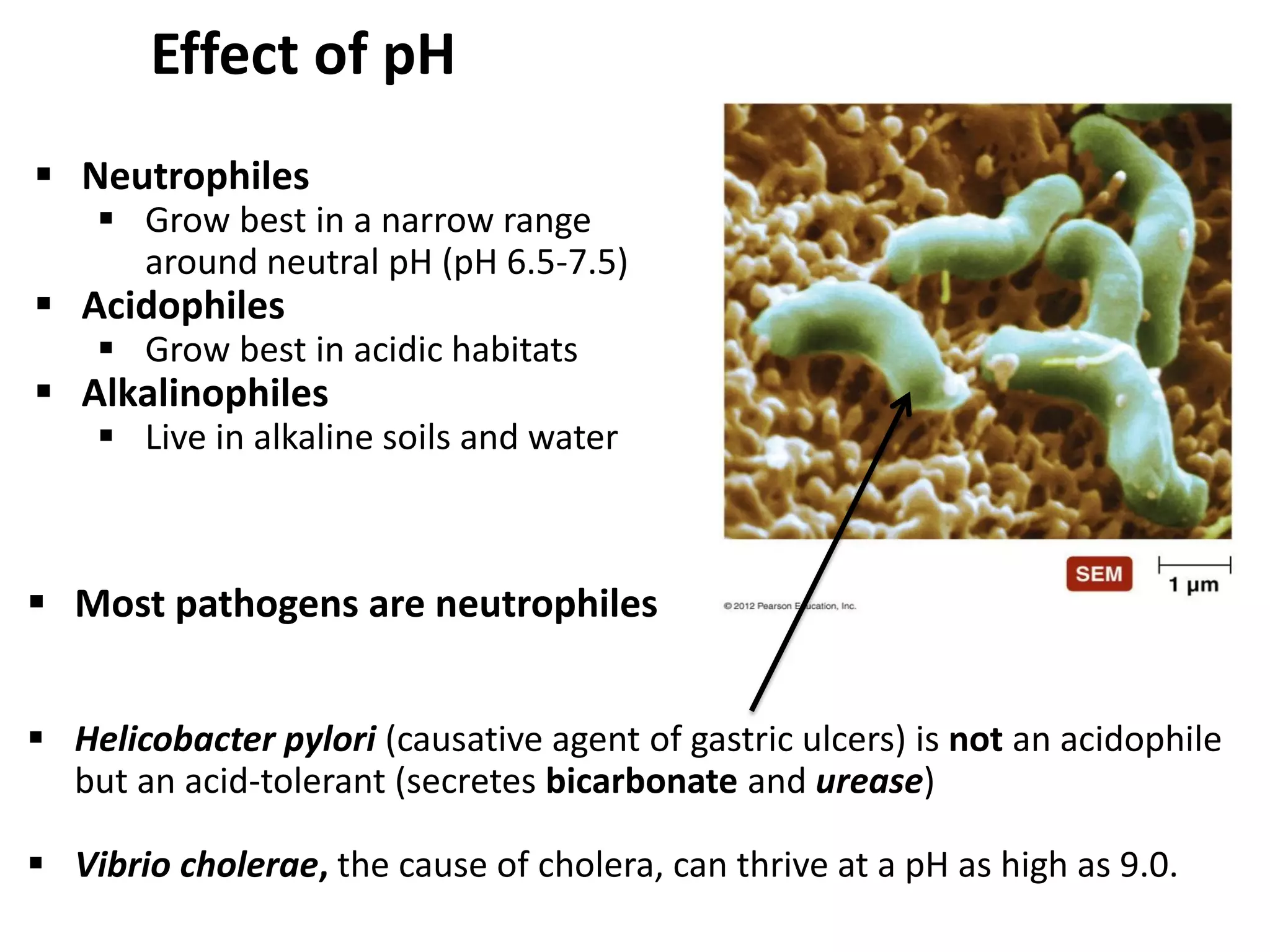
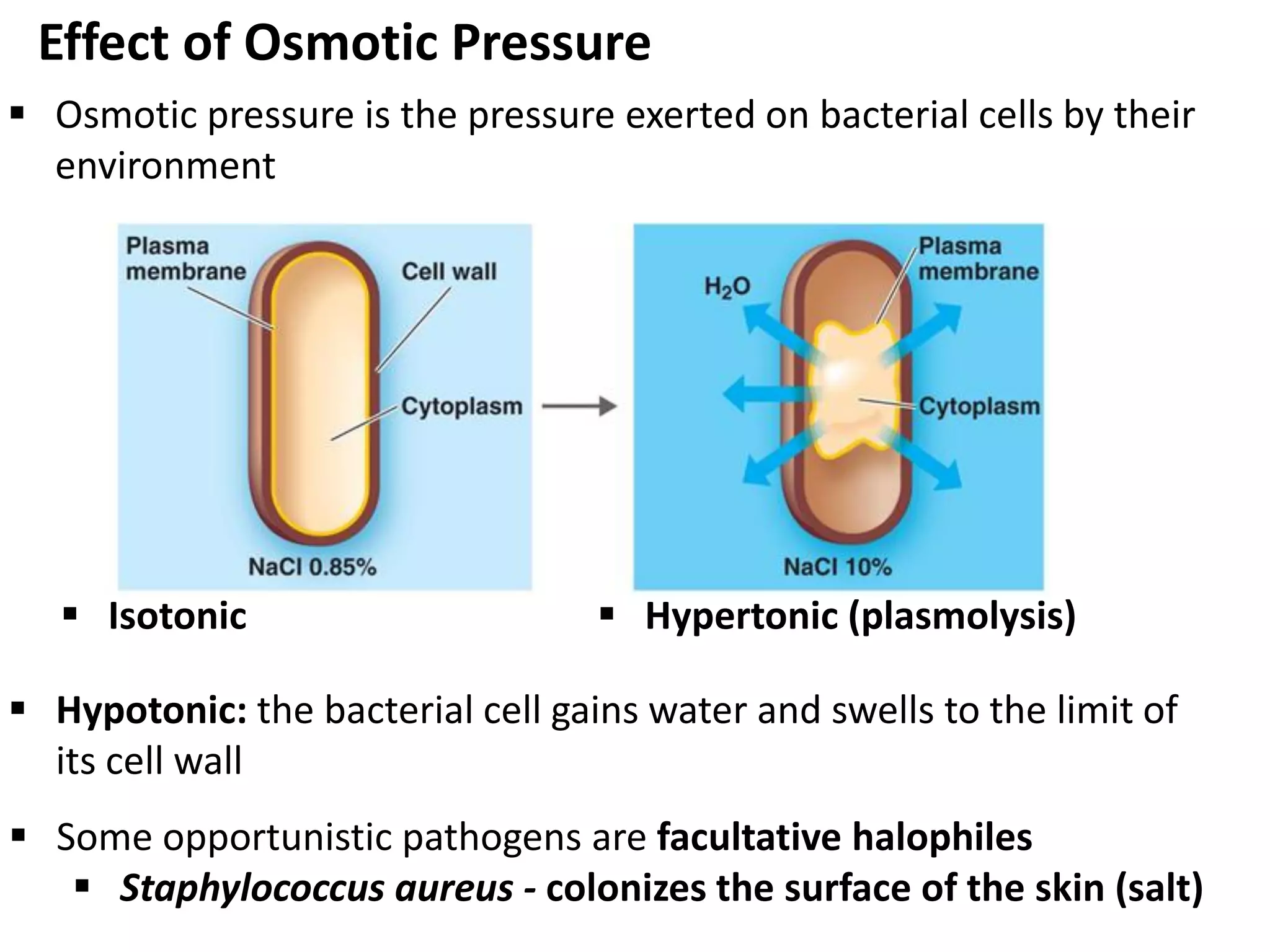
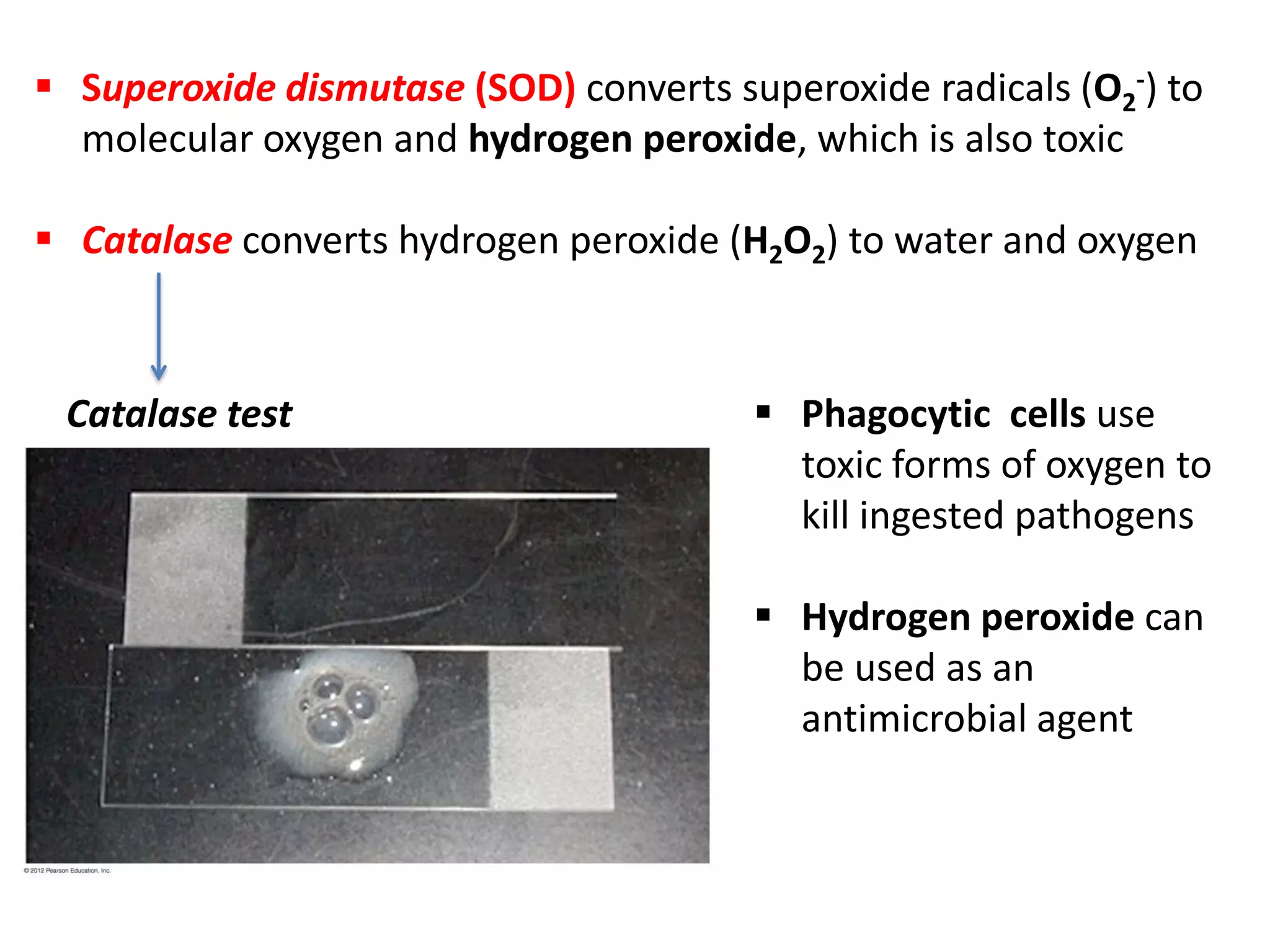
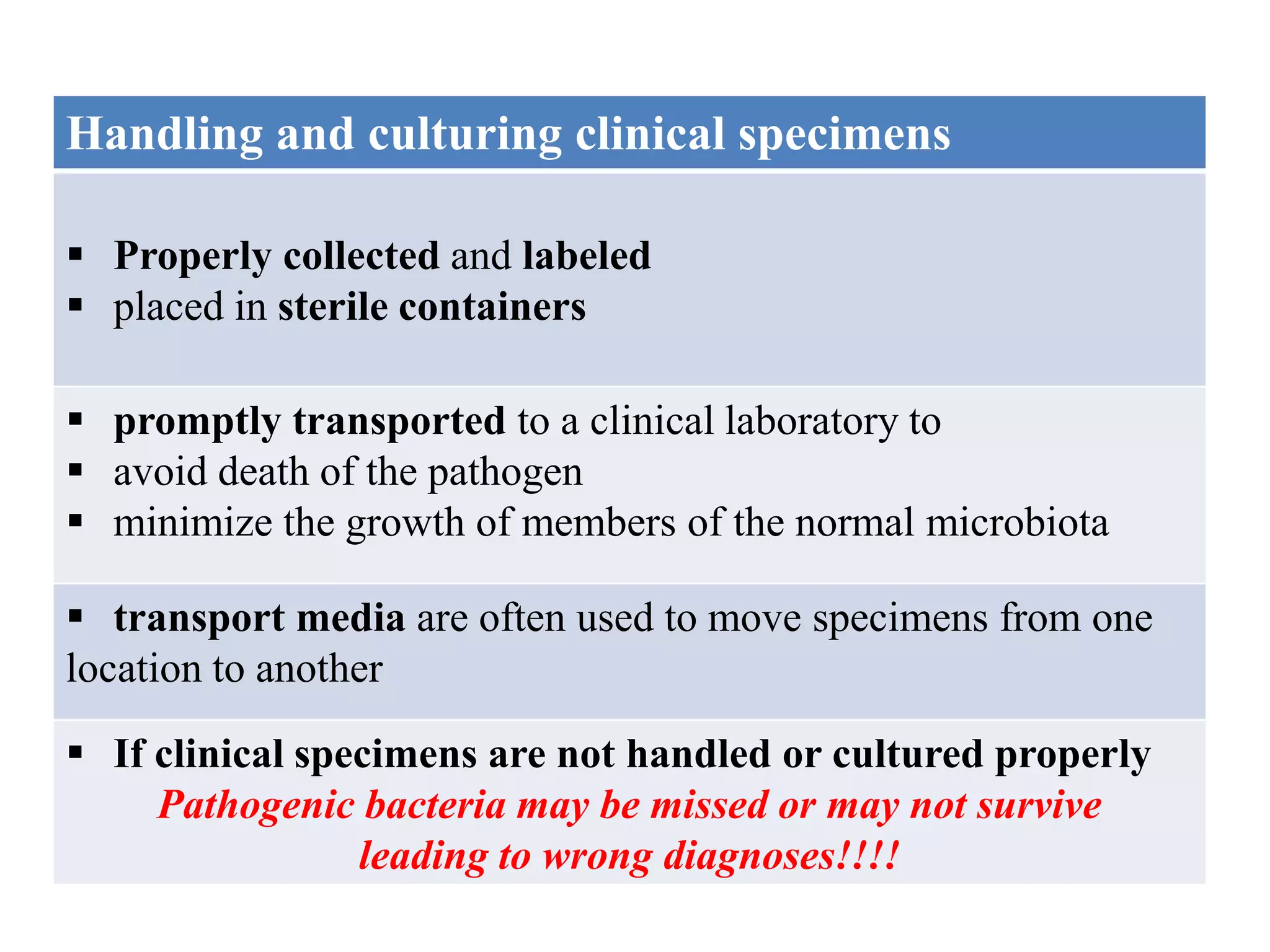
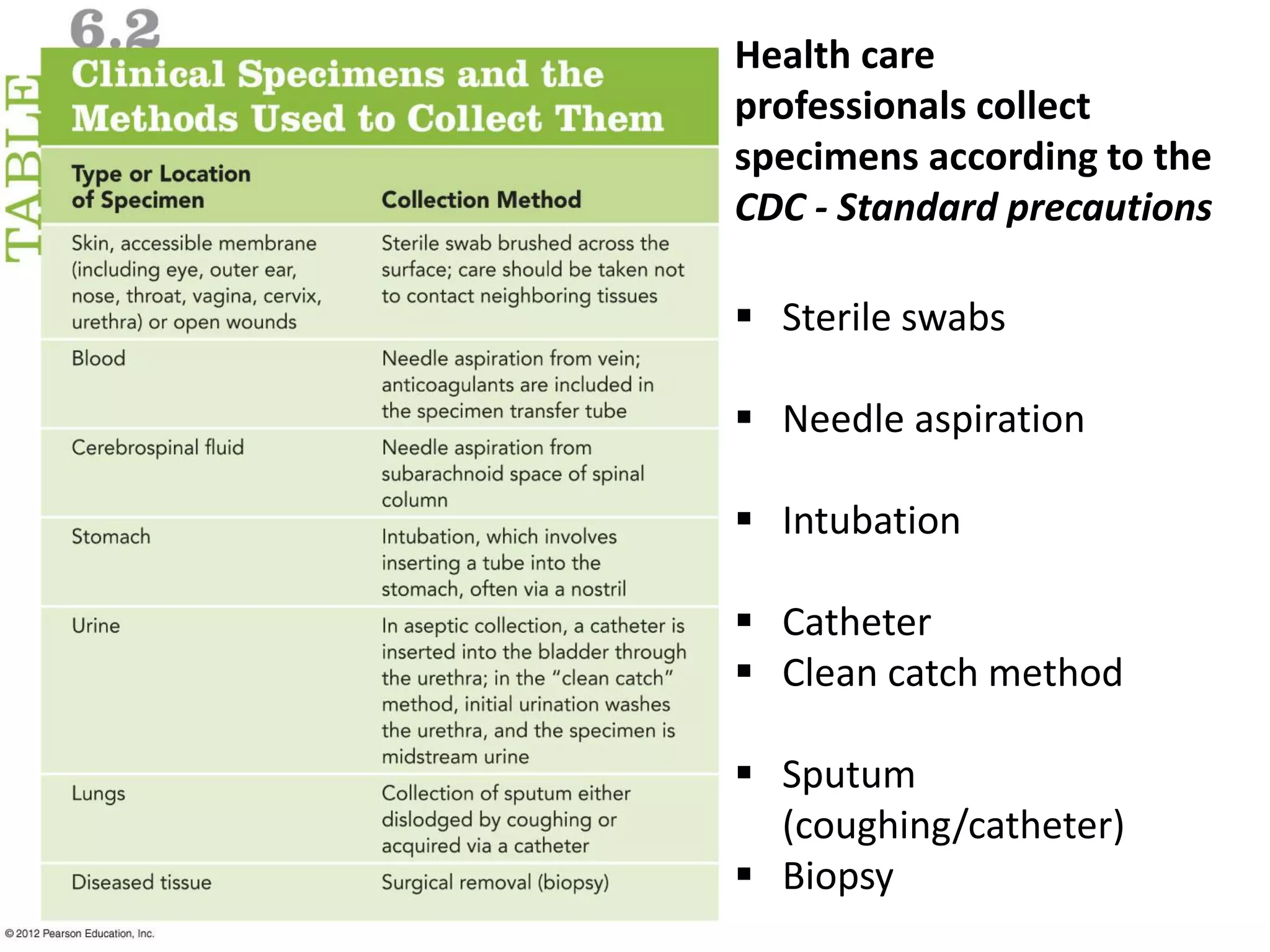
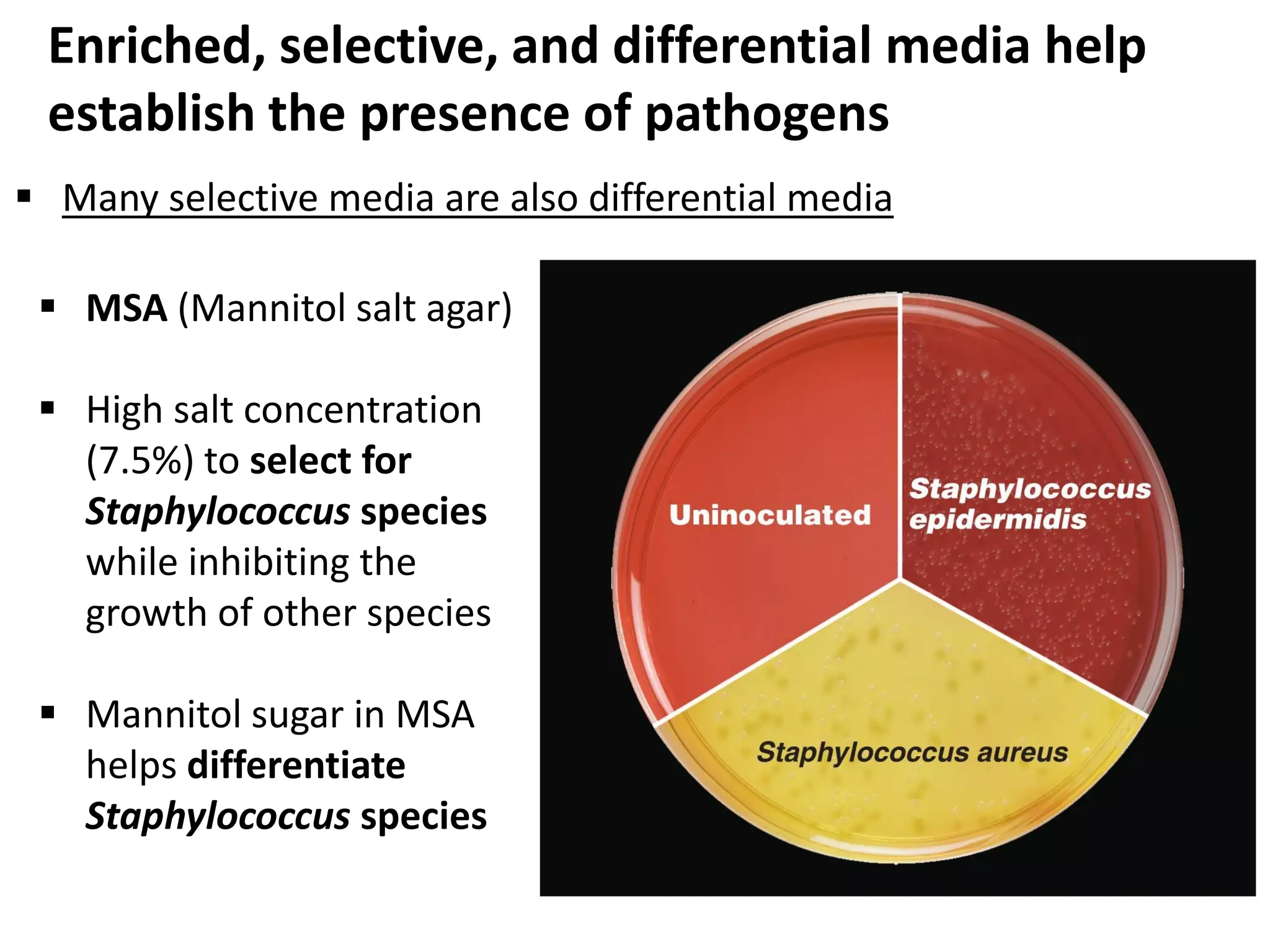
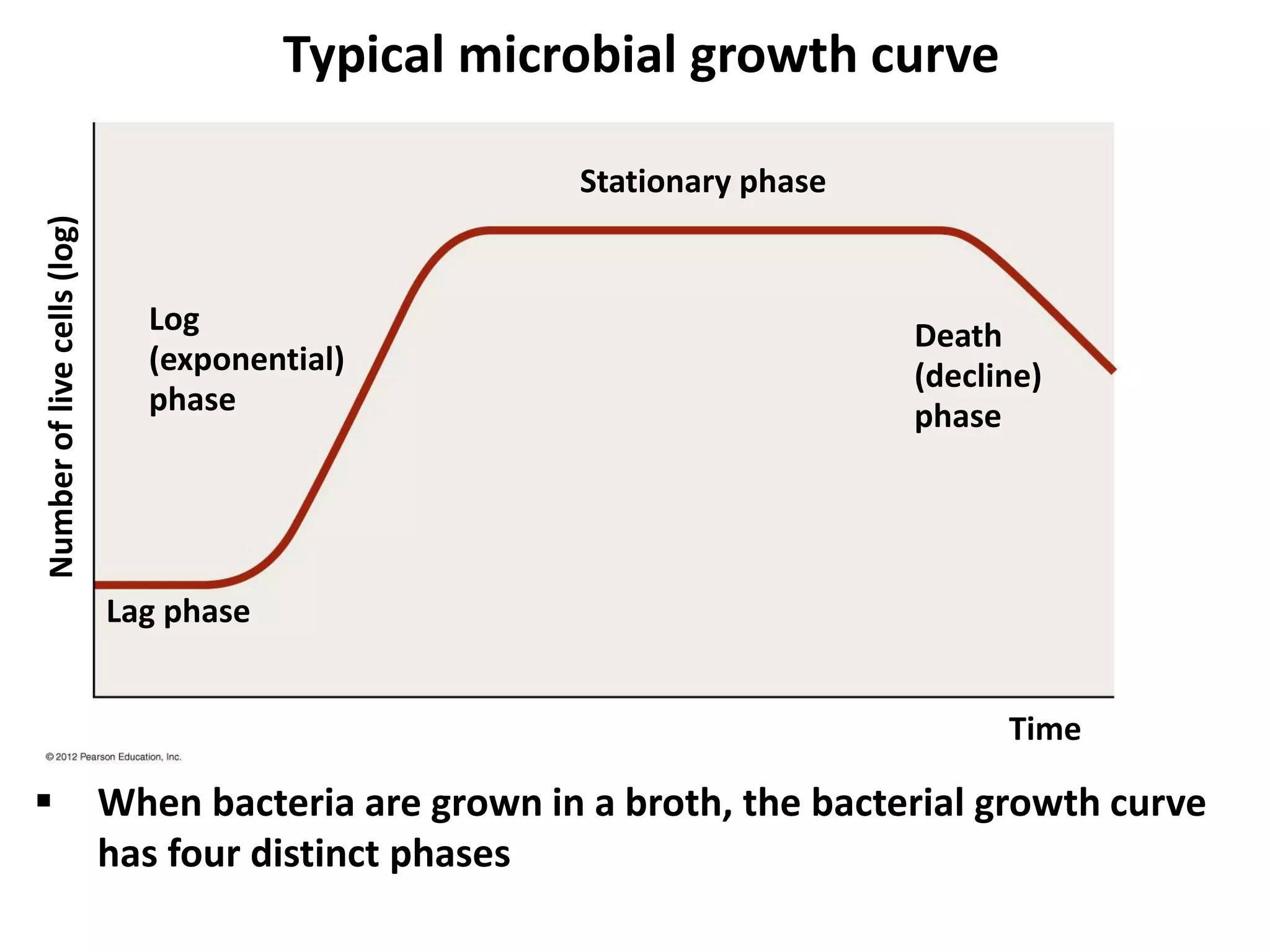
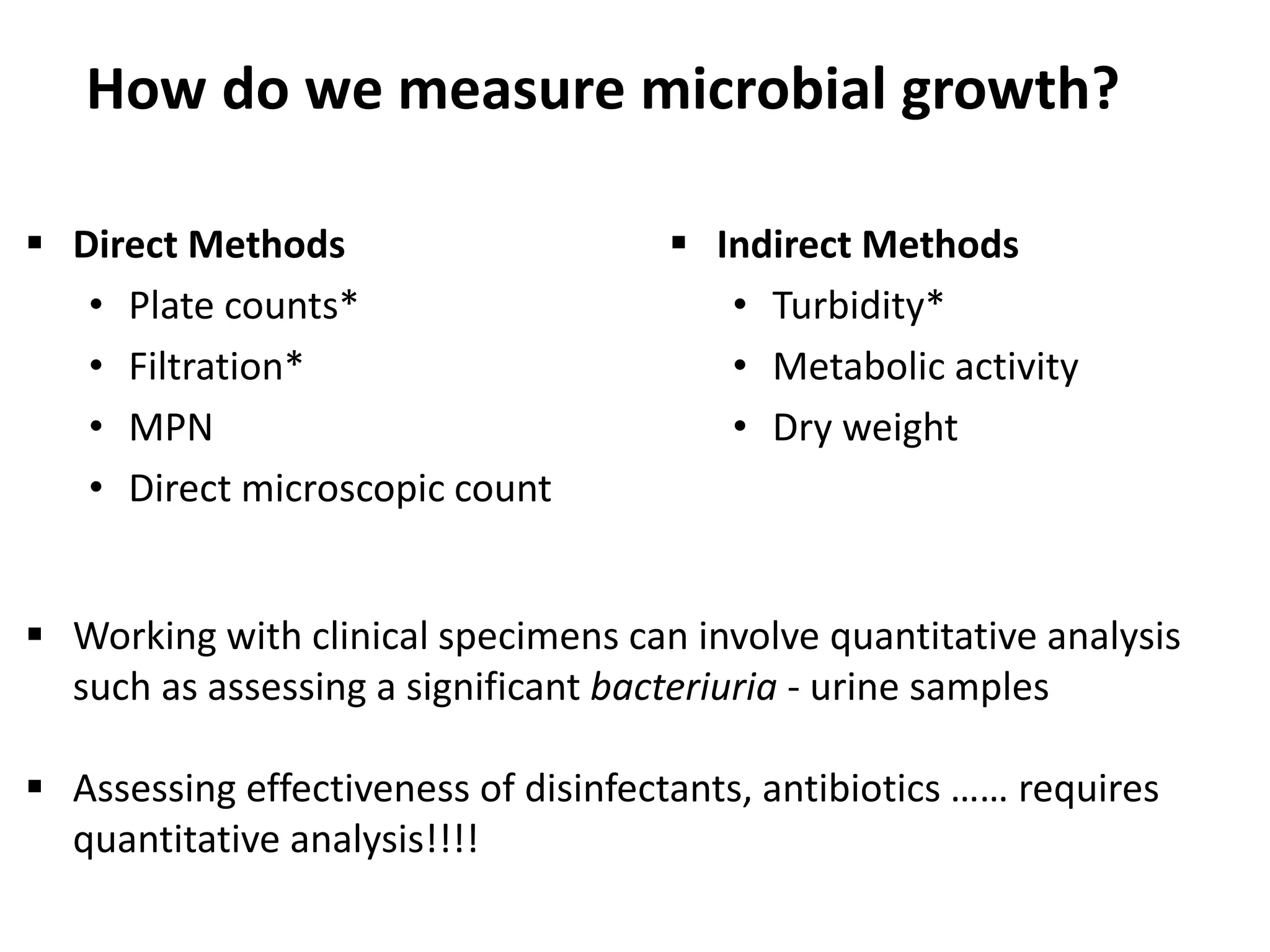
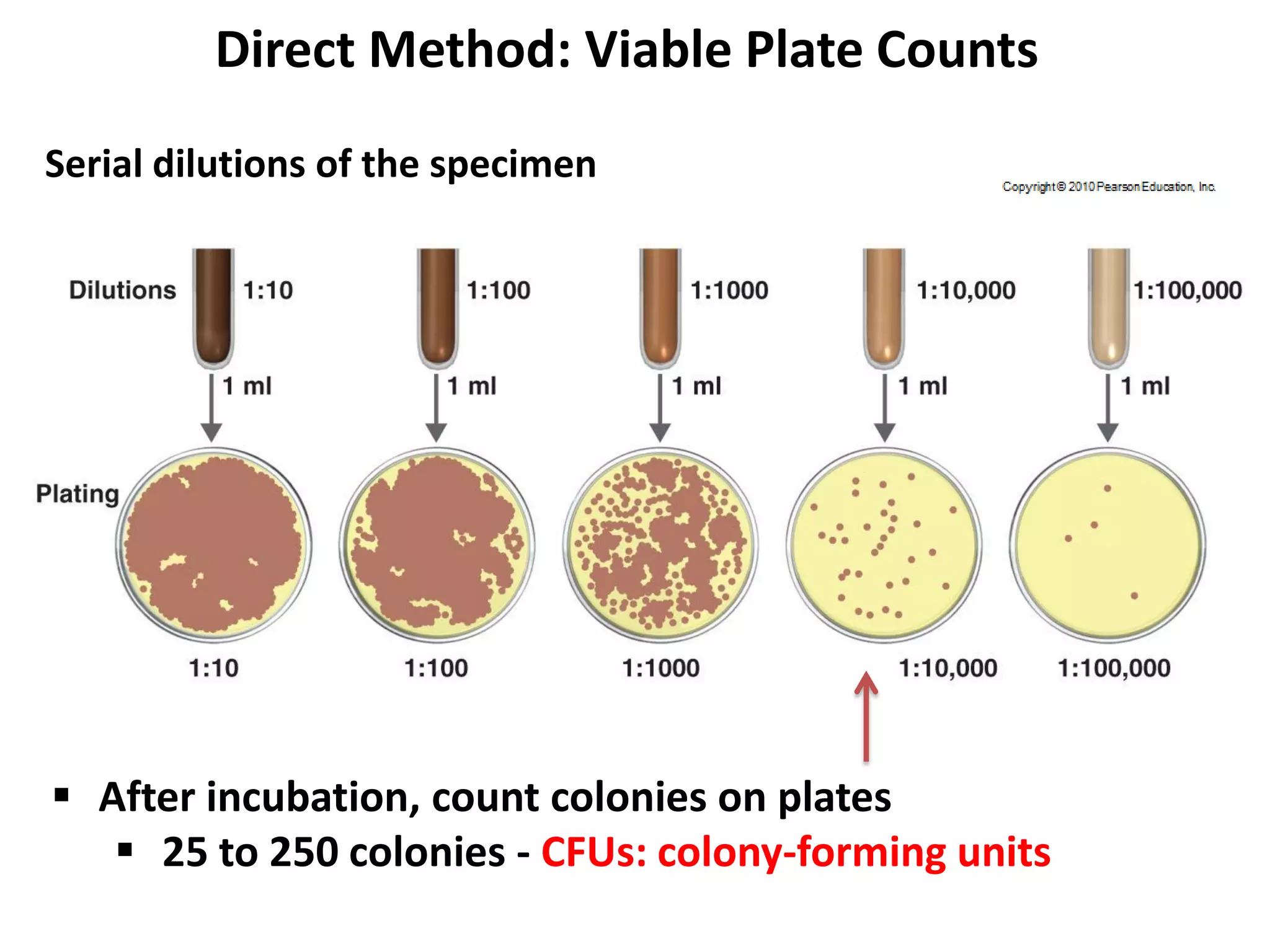
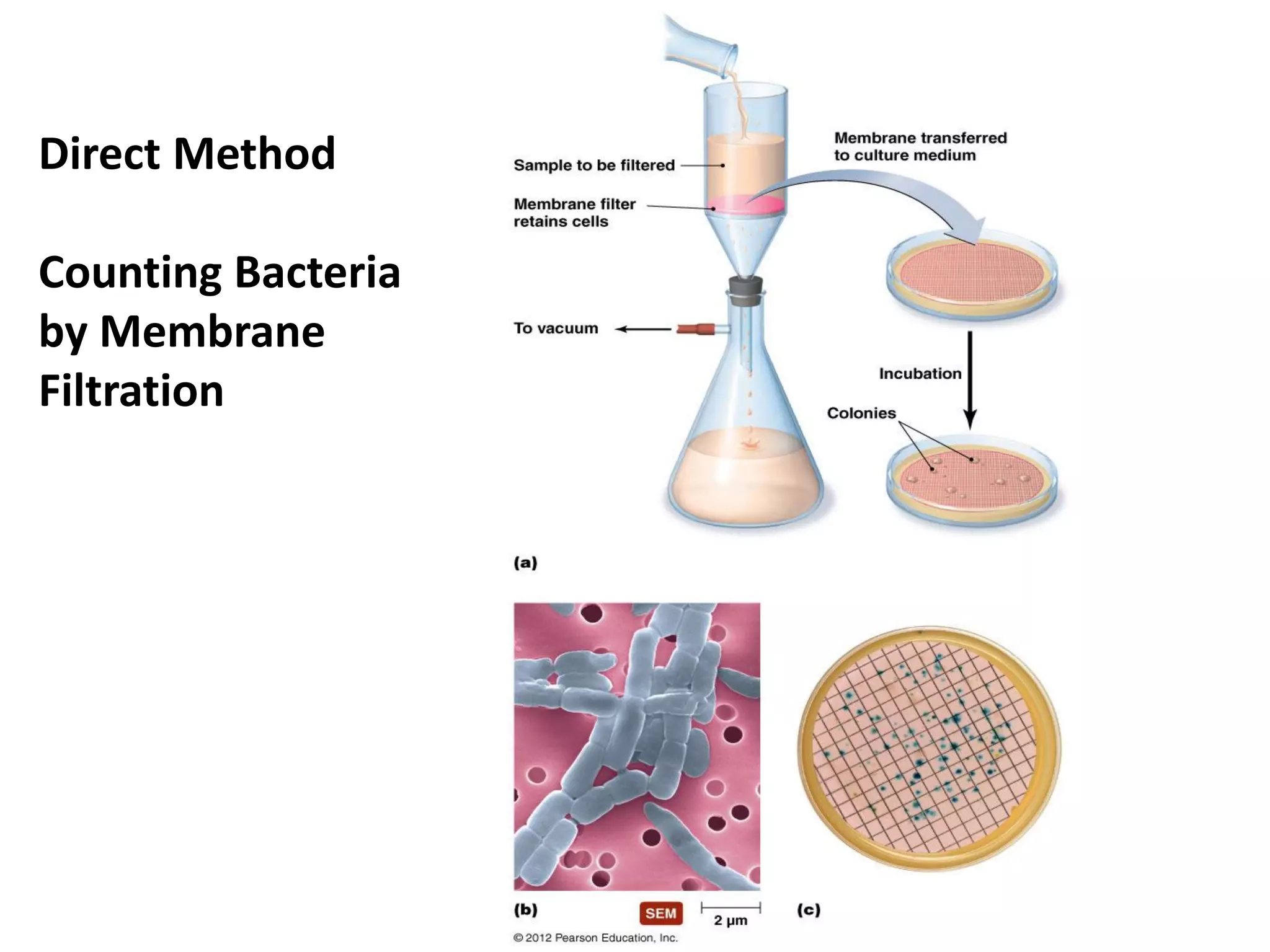
Microbial growth requires certain physical, chemical, and energy requirements to be met. Understanding these growth requirements allows us to control microbes, especially pathogens. Key growth factors include temperature, pH, oxygen levels, and nutrients. Different microbes have varying optimal temperature, pH, and oxygen ranges. Using specialized culture media that provide appropriate conditions enables isolation and growth of microbes from clinical specimens in the laboratory, facilitating identification of pathogens.