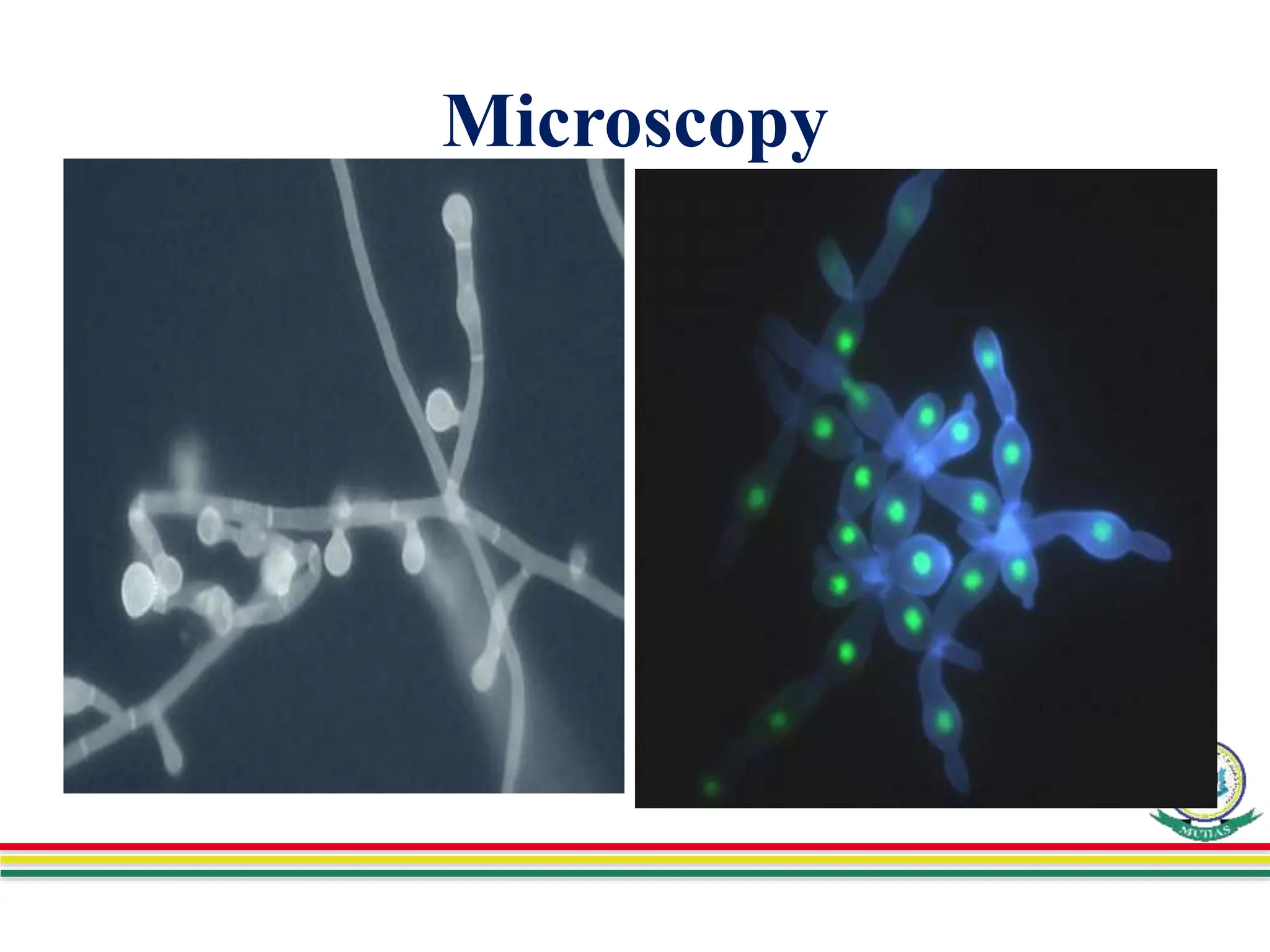
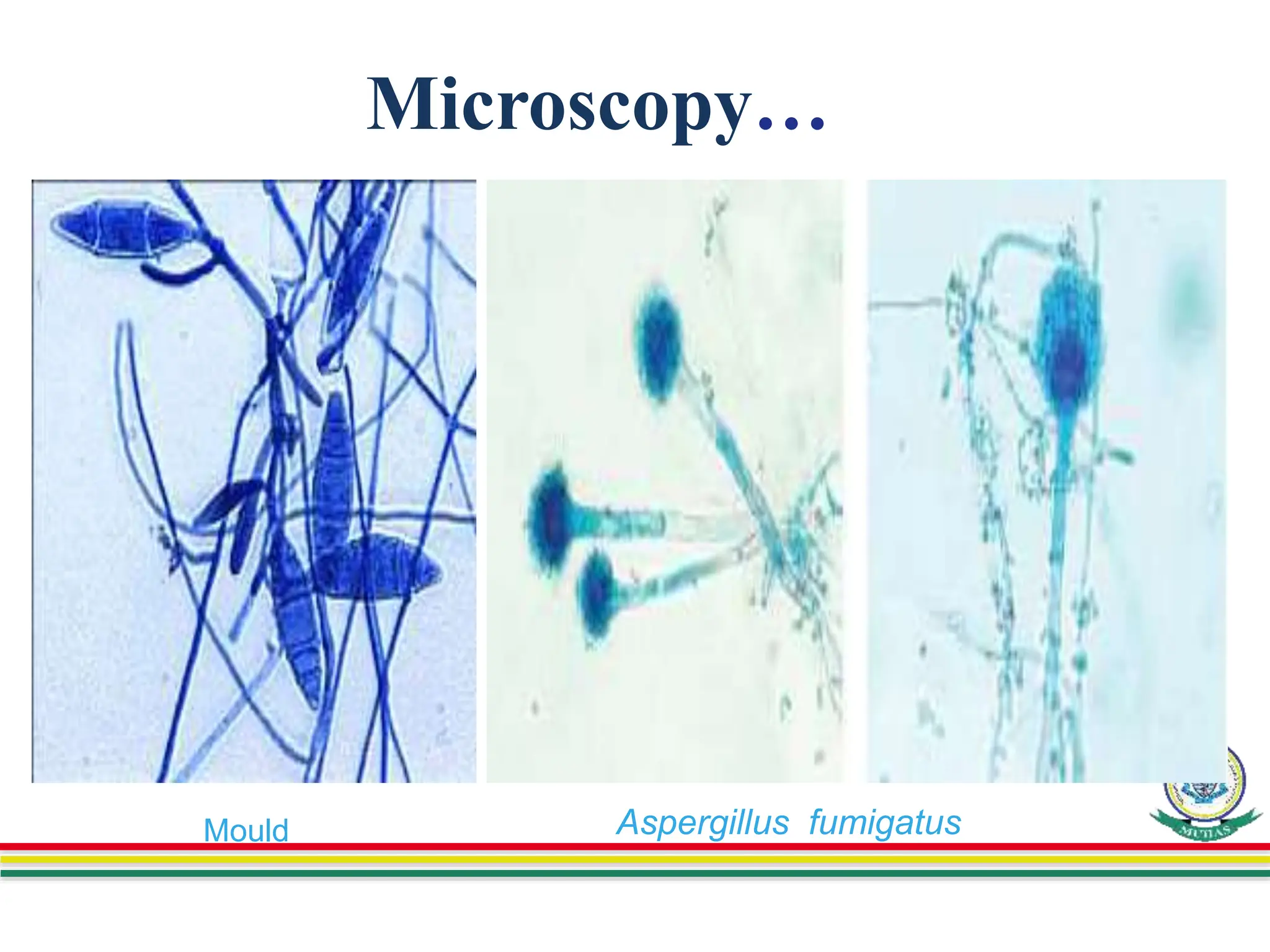
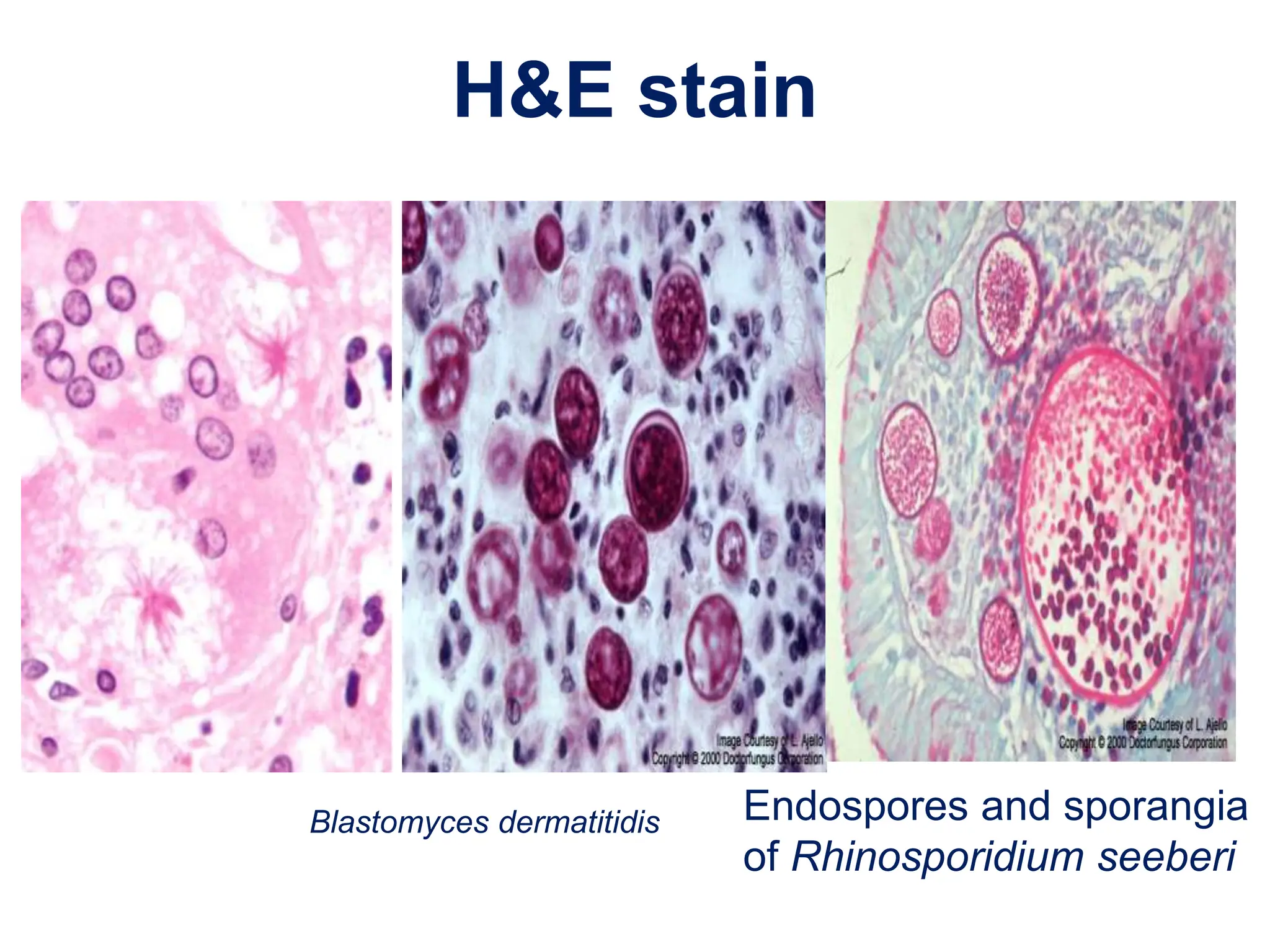
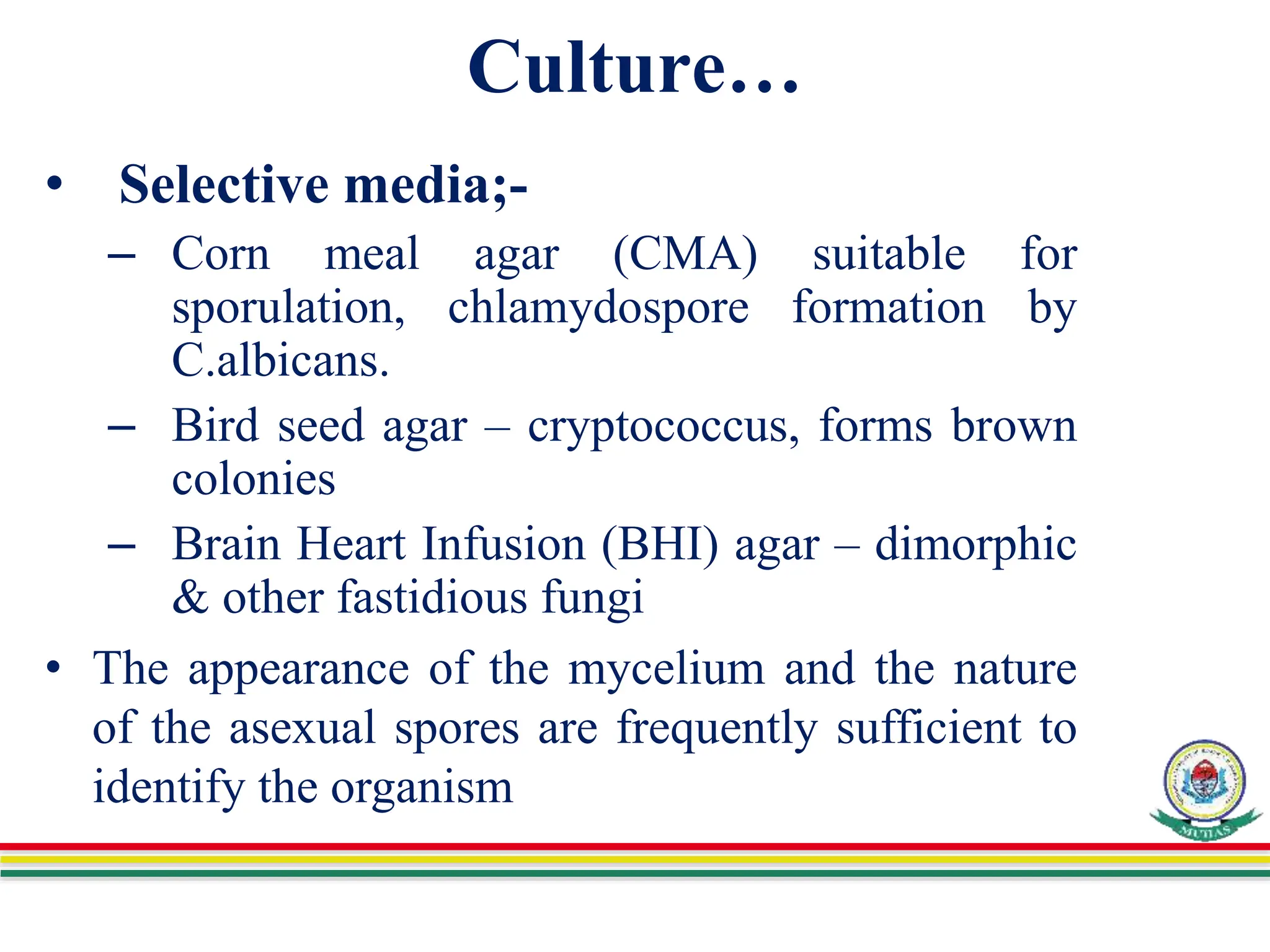
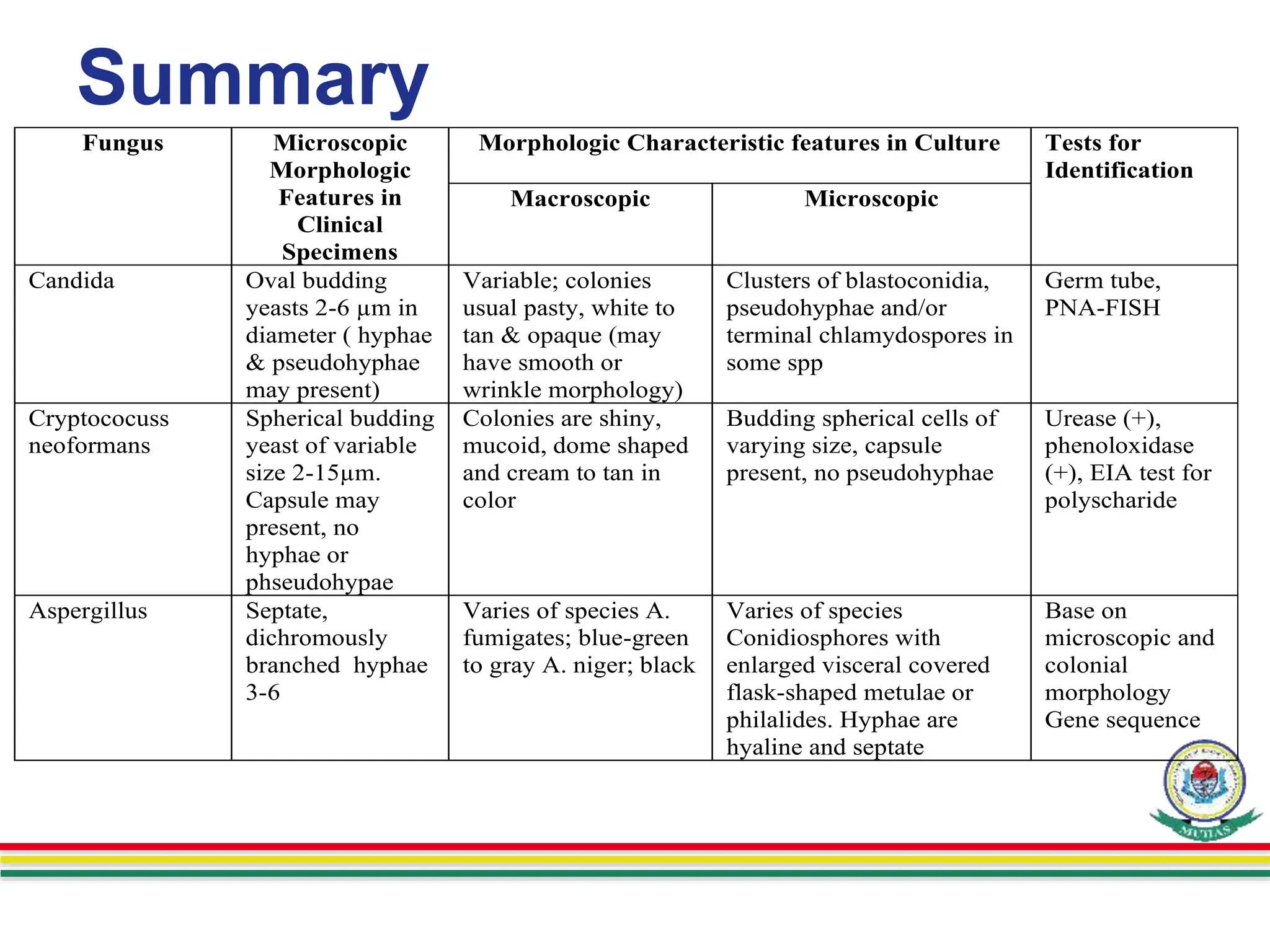
This document discusses various methods used to identify fungal infections, including microscopic, cultural, histopathological, and molecular approaches. Microscopic examination methods include wet mount preparations using potassium hydroxide or calcofluor white stain to visualize fungal structures. Histopathological stains like Gomori methenamine-silver and periodic acid-Schiff are used to identify fungi in tissue sections. Culture allows identification based on fungal morphology and biochemical tests. Molecular methods like PCR are also used for diagnosis. Dermatophytes causing ringworm are identified by their microscopic morphology and growth characteristics.