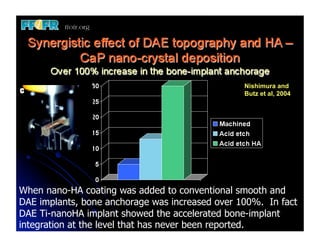
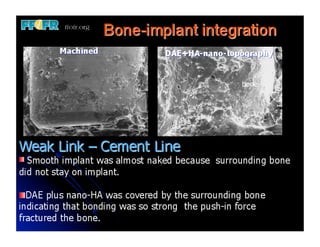
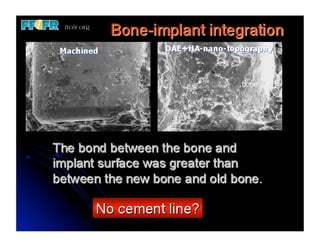
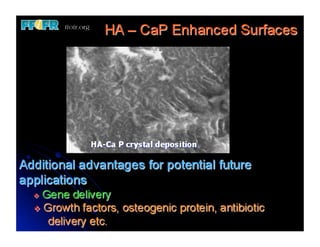
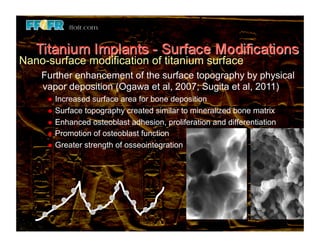
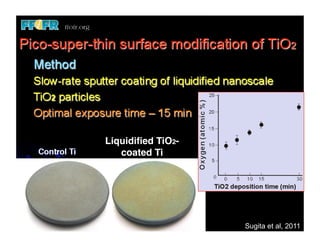
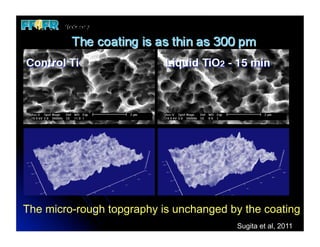
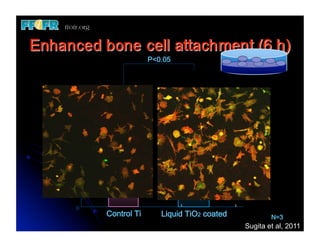
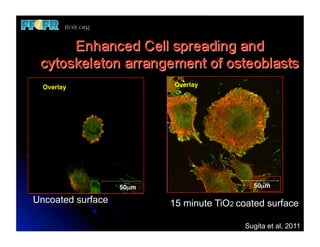
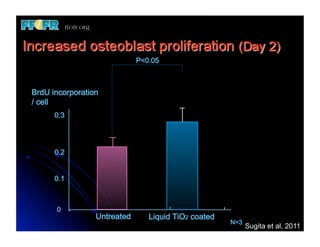
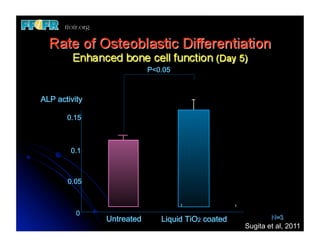
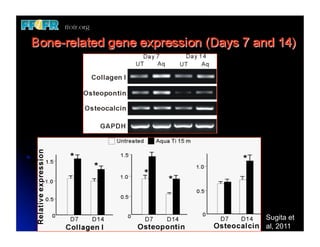
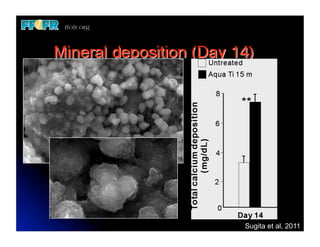
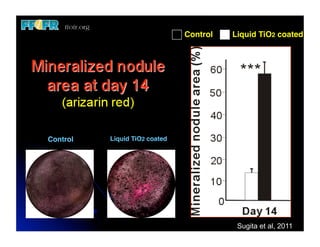
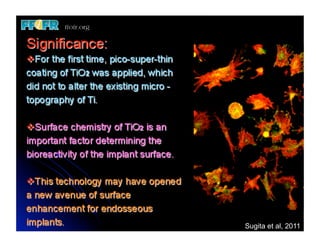
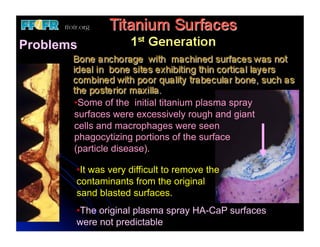
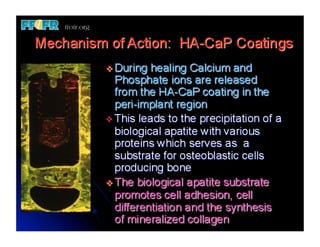
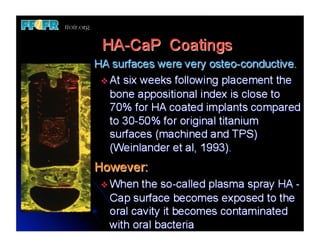
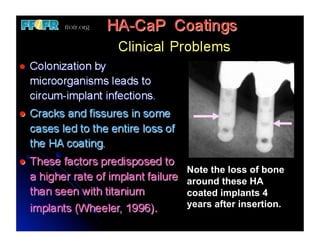
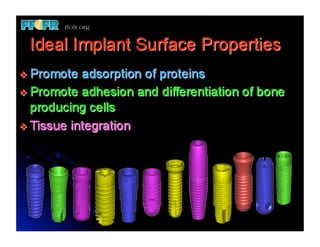
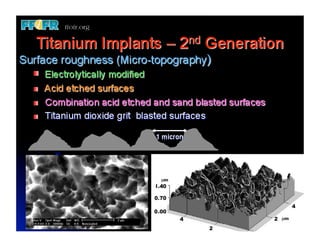
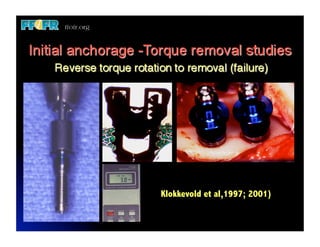
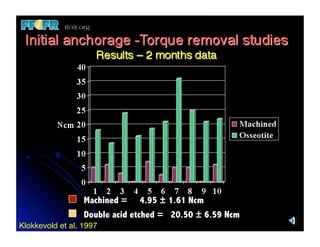
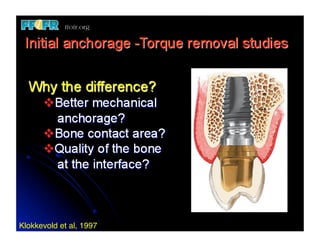
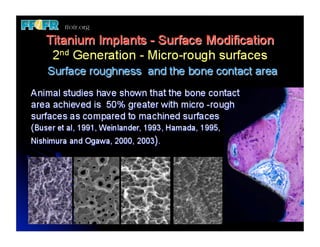
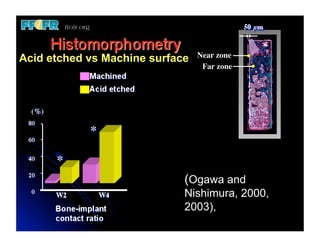
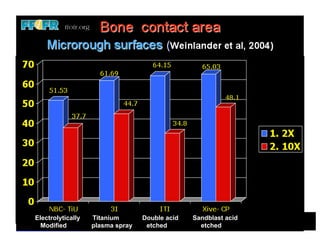
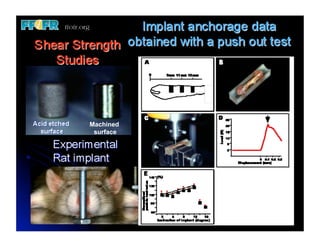
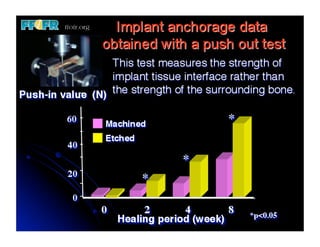
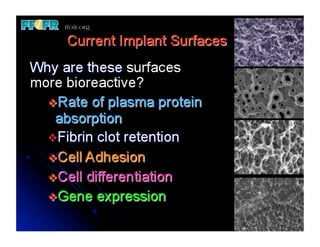
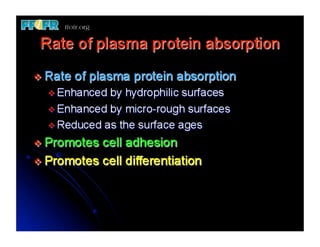
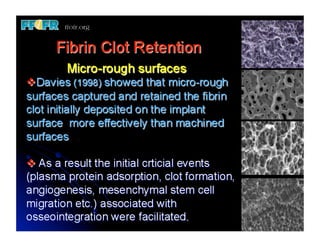
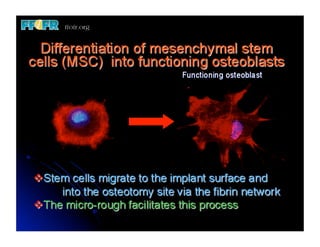
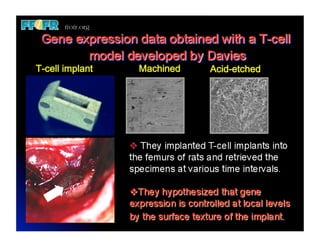
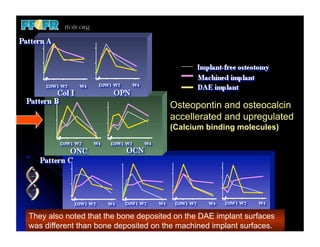
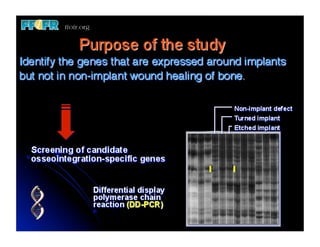
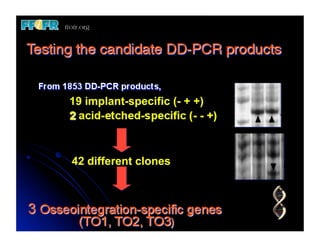
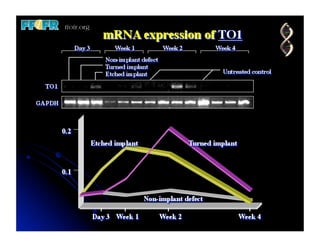
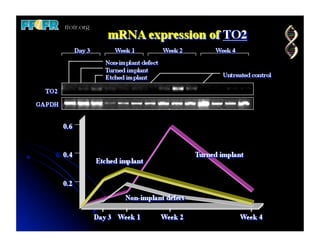
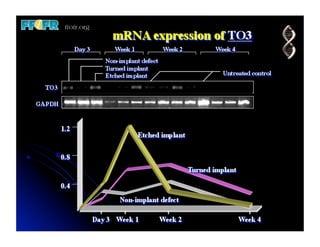
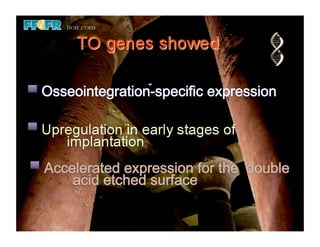
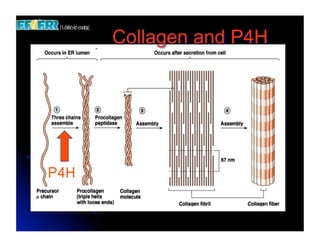
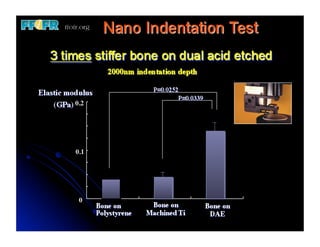
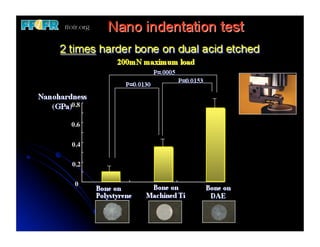
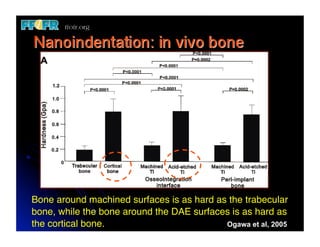
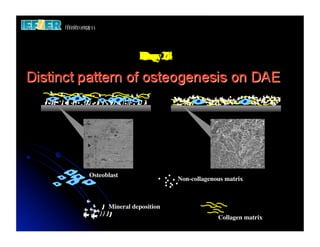
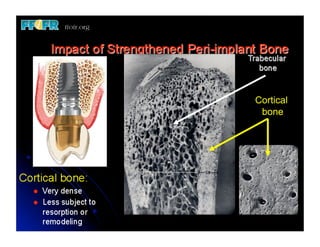
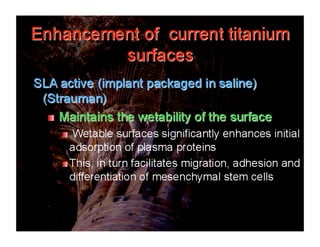
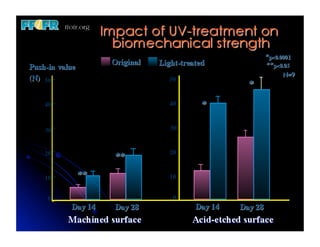
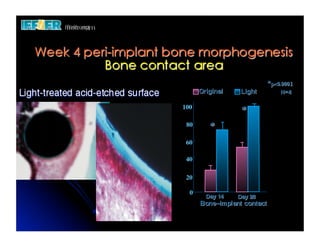
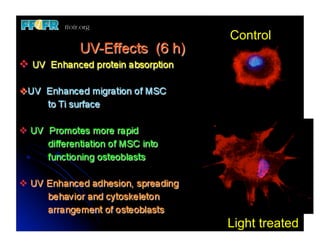
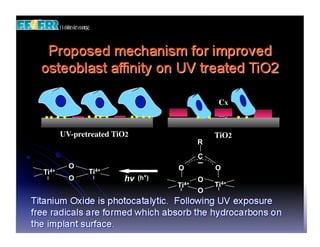
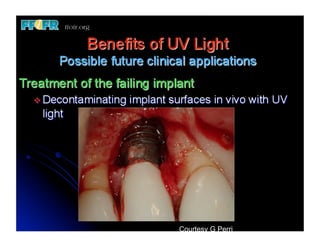
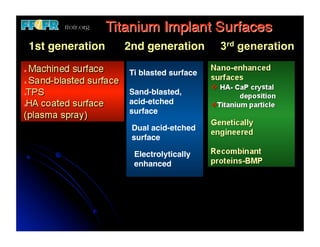
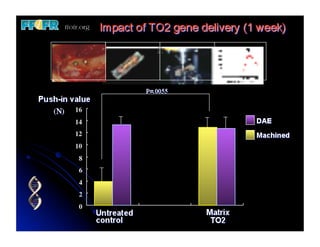
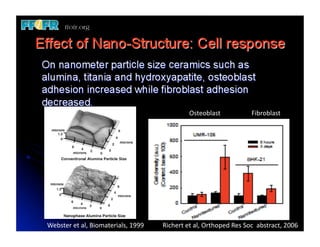
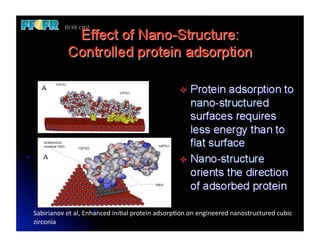
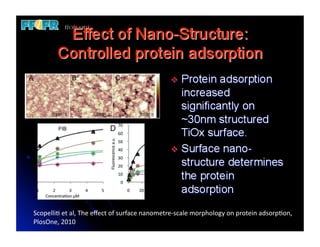
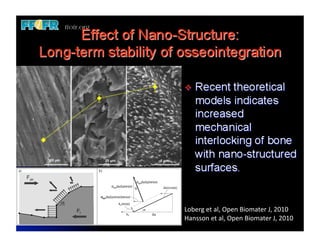
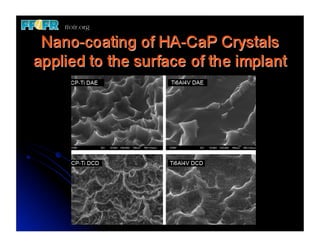
The document discusses problems with initial titanium plasma spray implant surfaces and issues removing contaminants from sandblasted surfaces. It then summarizes several studies showing double acid-etched implant surfaces promote faster and greater bone formation compared to machined surfaces. Specifically, bone deposited on double acid-etched surfaces is harder, with collagen organized differently, and genes related to bone formation are upregulated faster. Coating implant surfaces with nanoscale hydroxyapatite or liquid titanium dioxide further enhances bone formation and implant integration.






























![Chemical Bonding?
Machined Ti
DAE Ti
DAE Ti-nanoHA
Shear strength at 2 wk
S=F/A [N/mm2]](https://image.slidesharecdn.com/2-recentadvancesinimplantsurfacescience-120108191009-phpapp01/85/2-recent-advances-in-implant-surface-science-80-320.jpg)