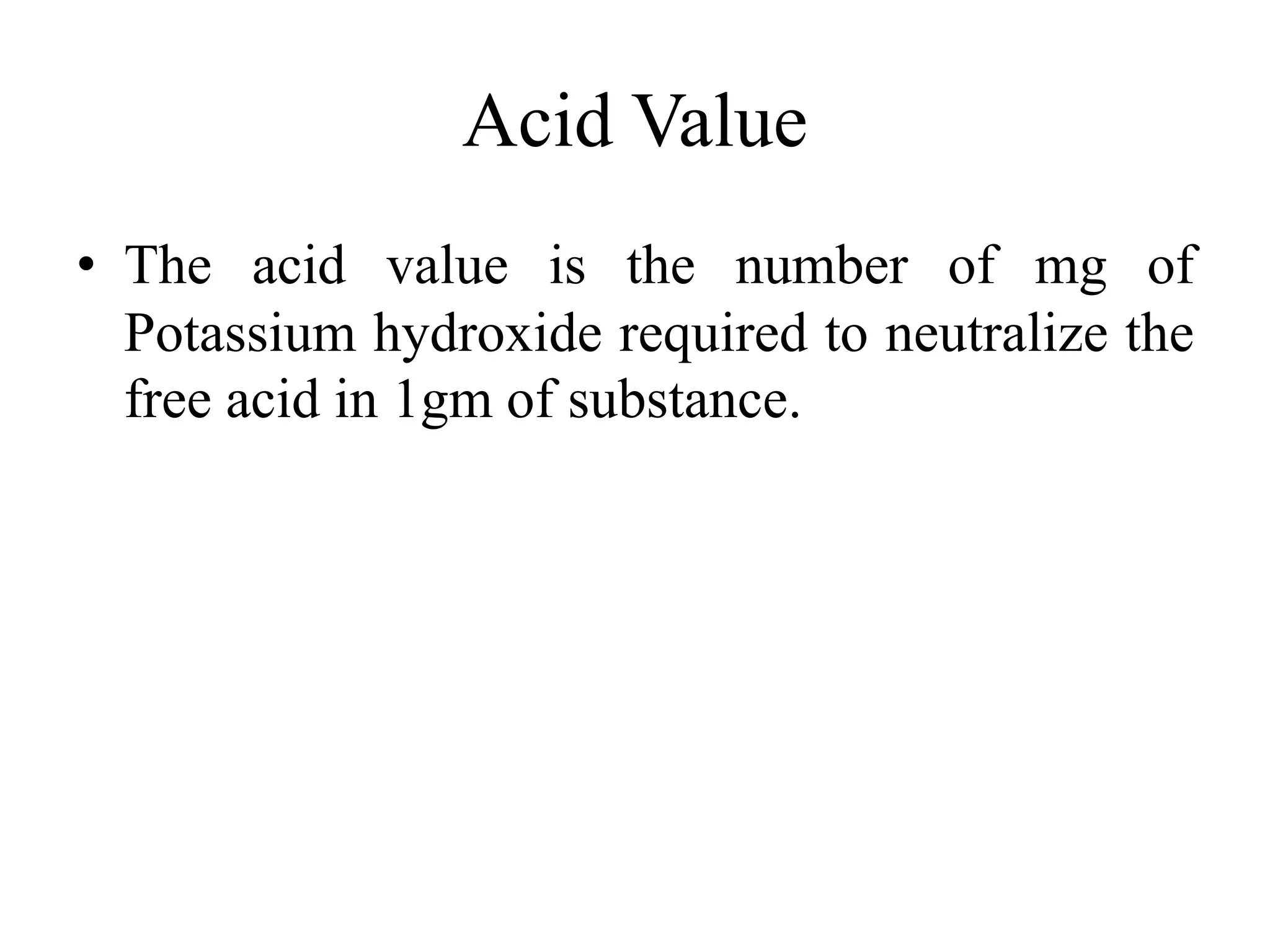
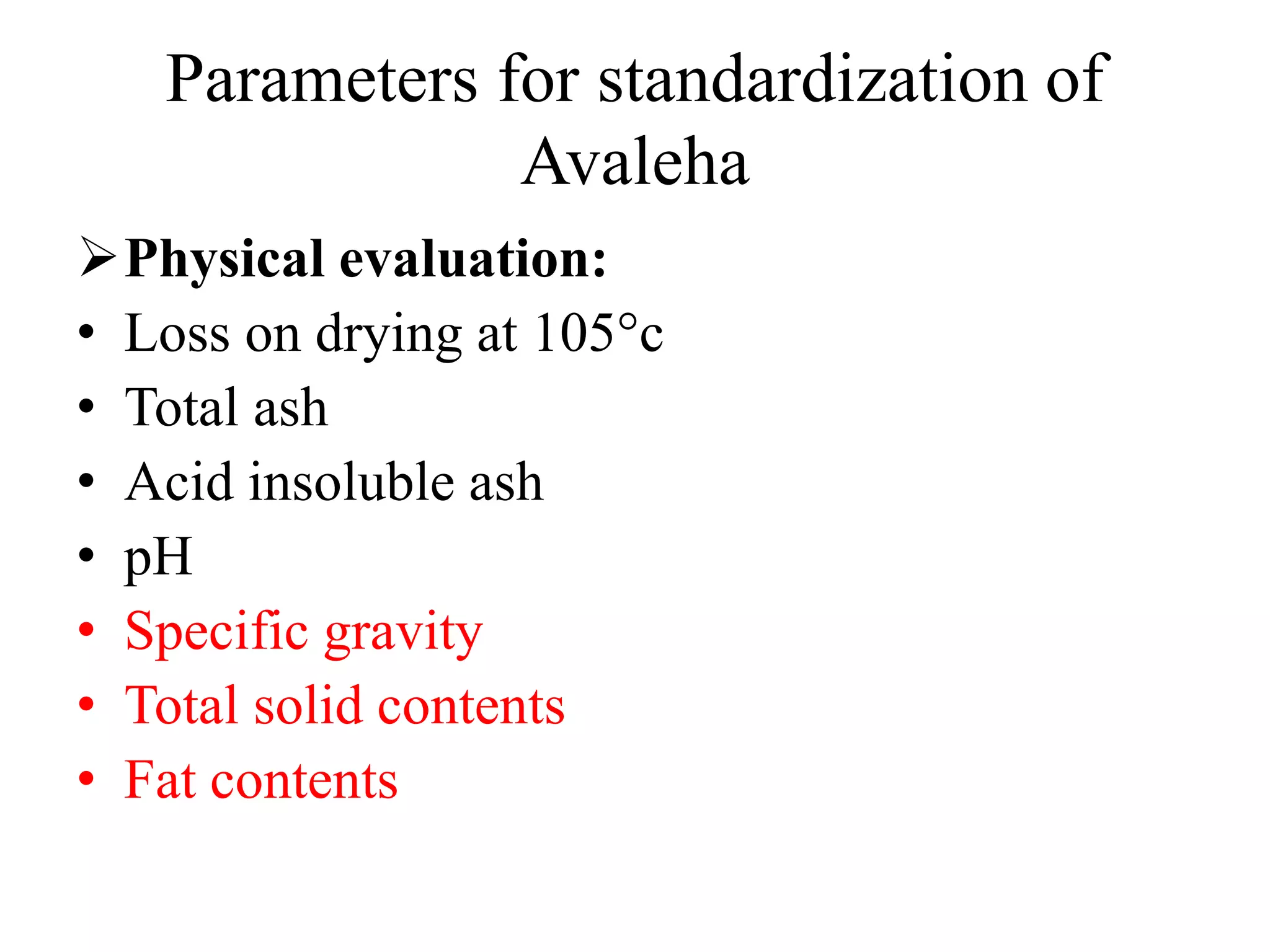
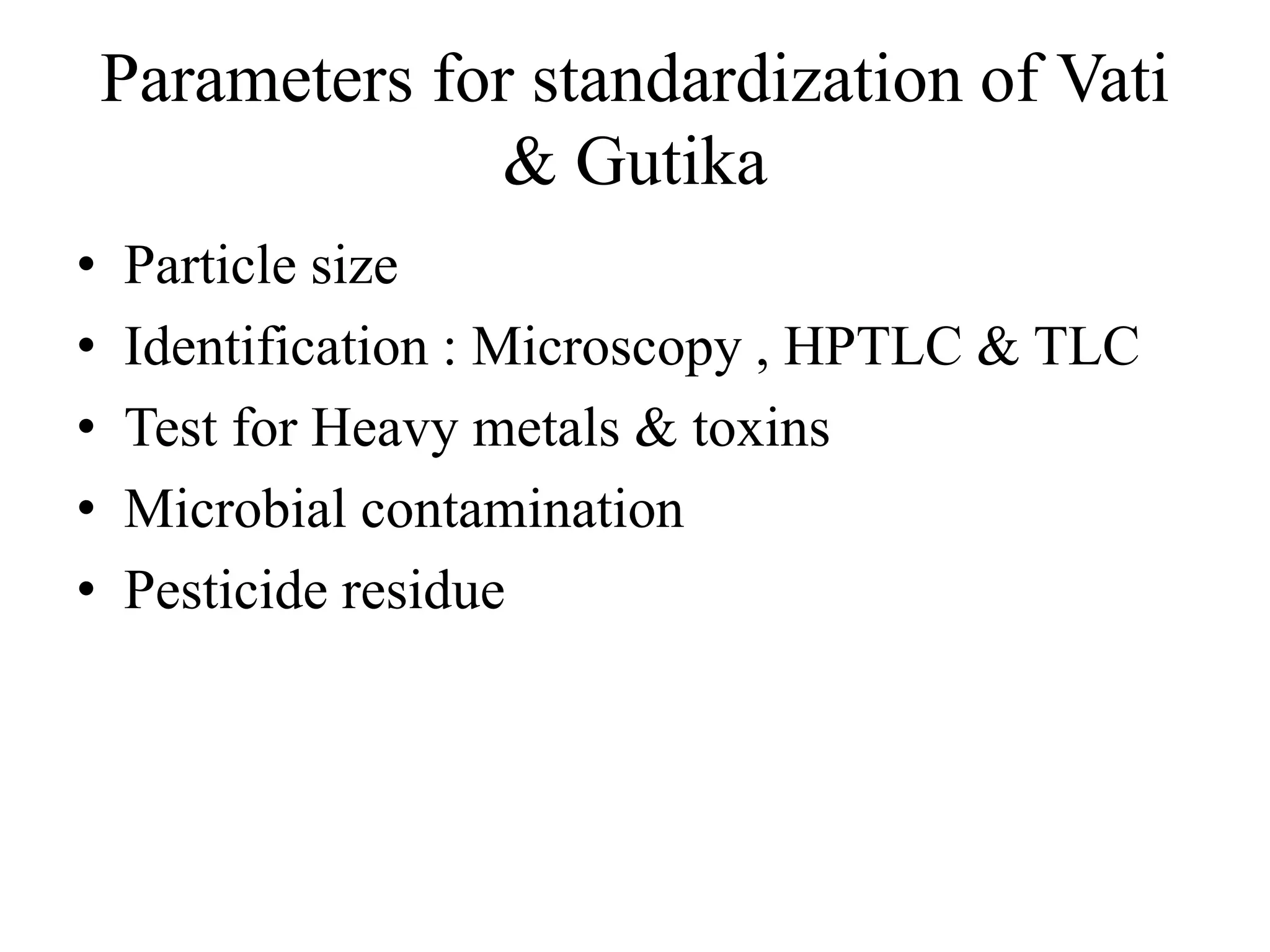
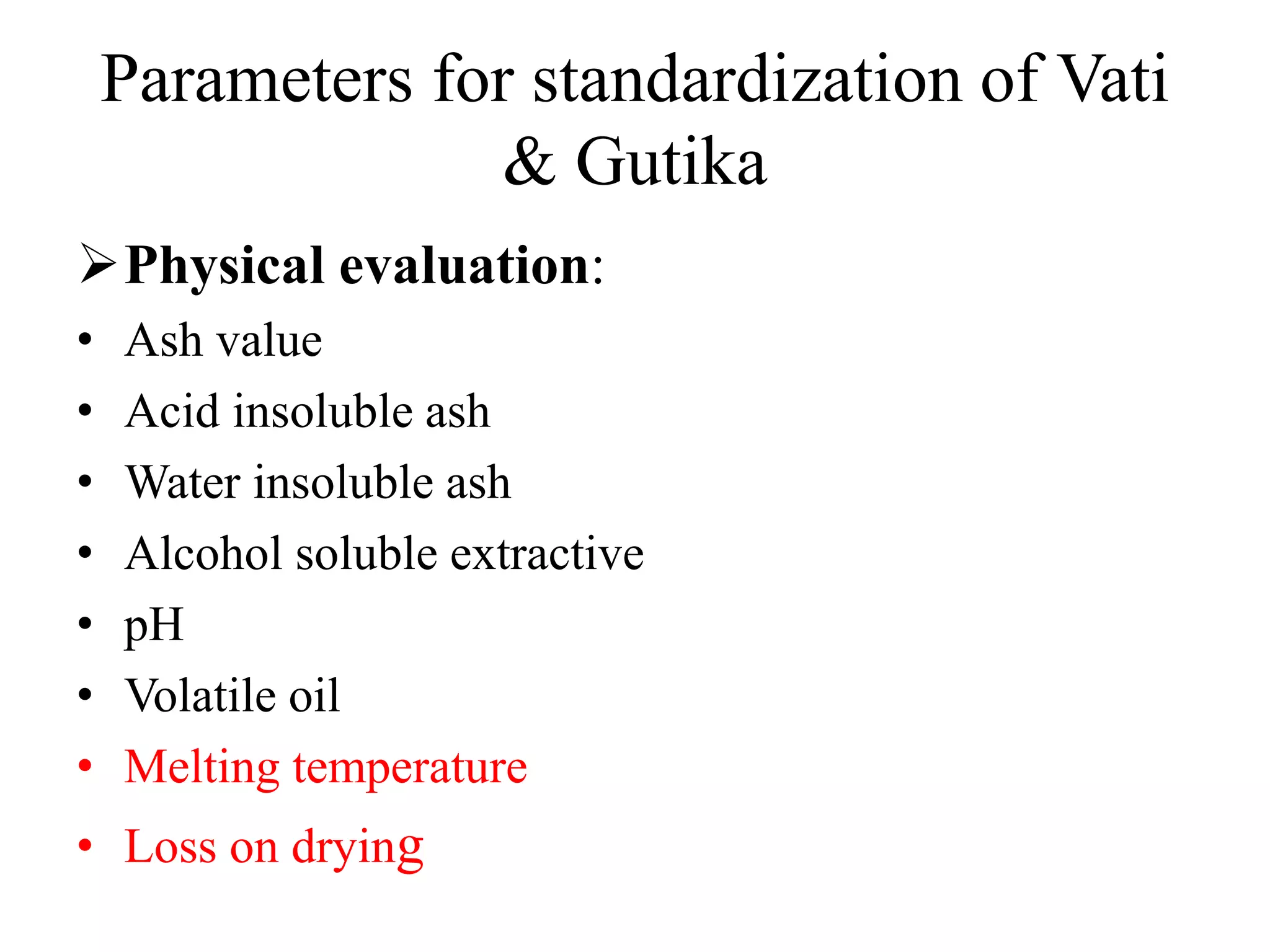
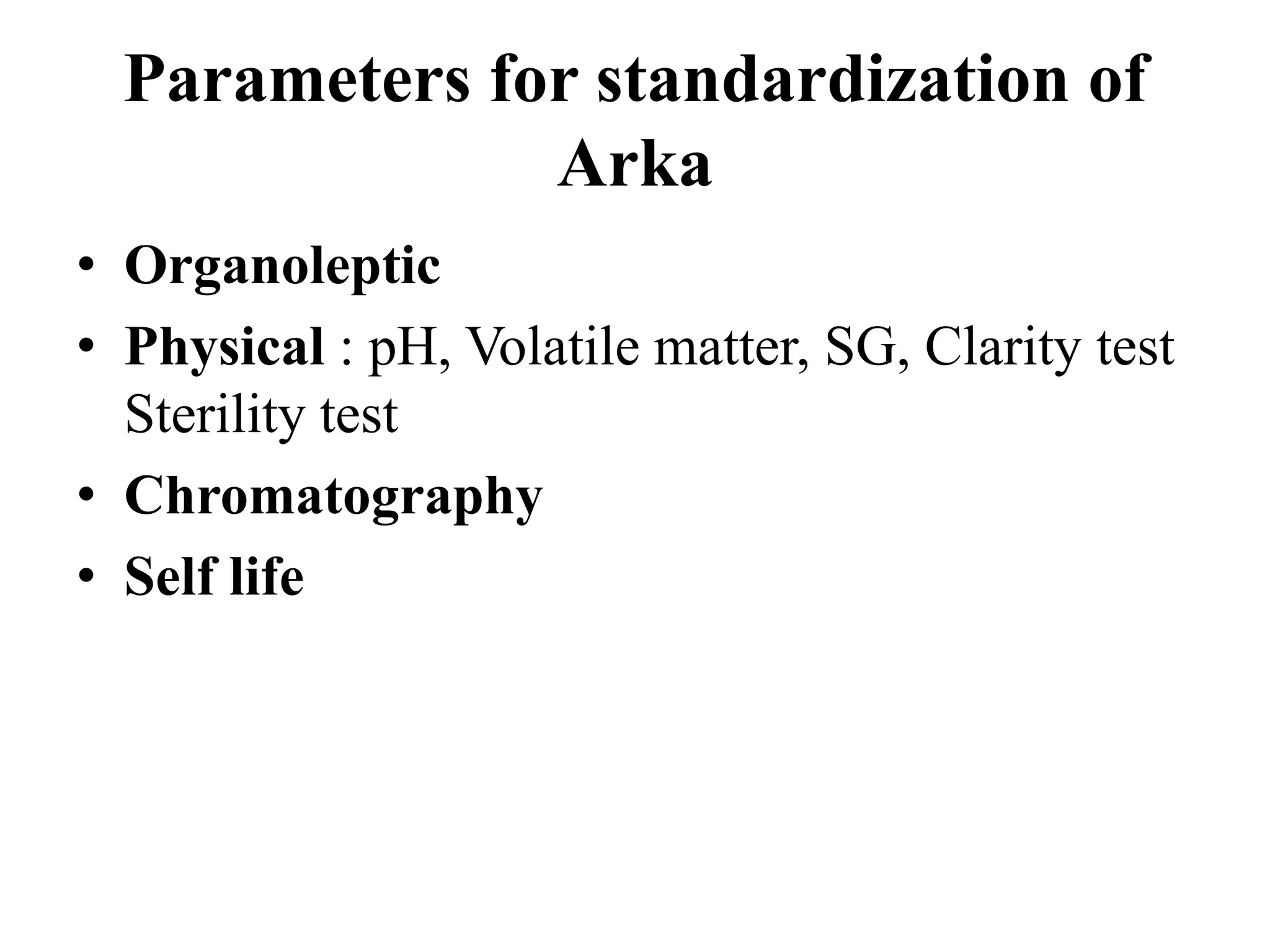
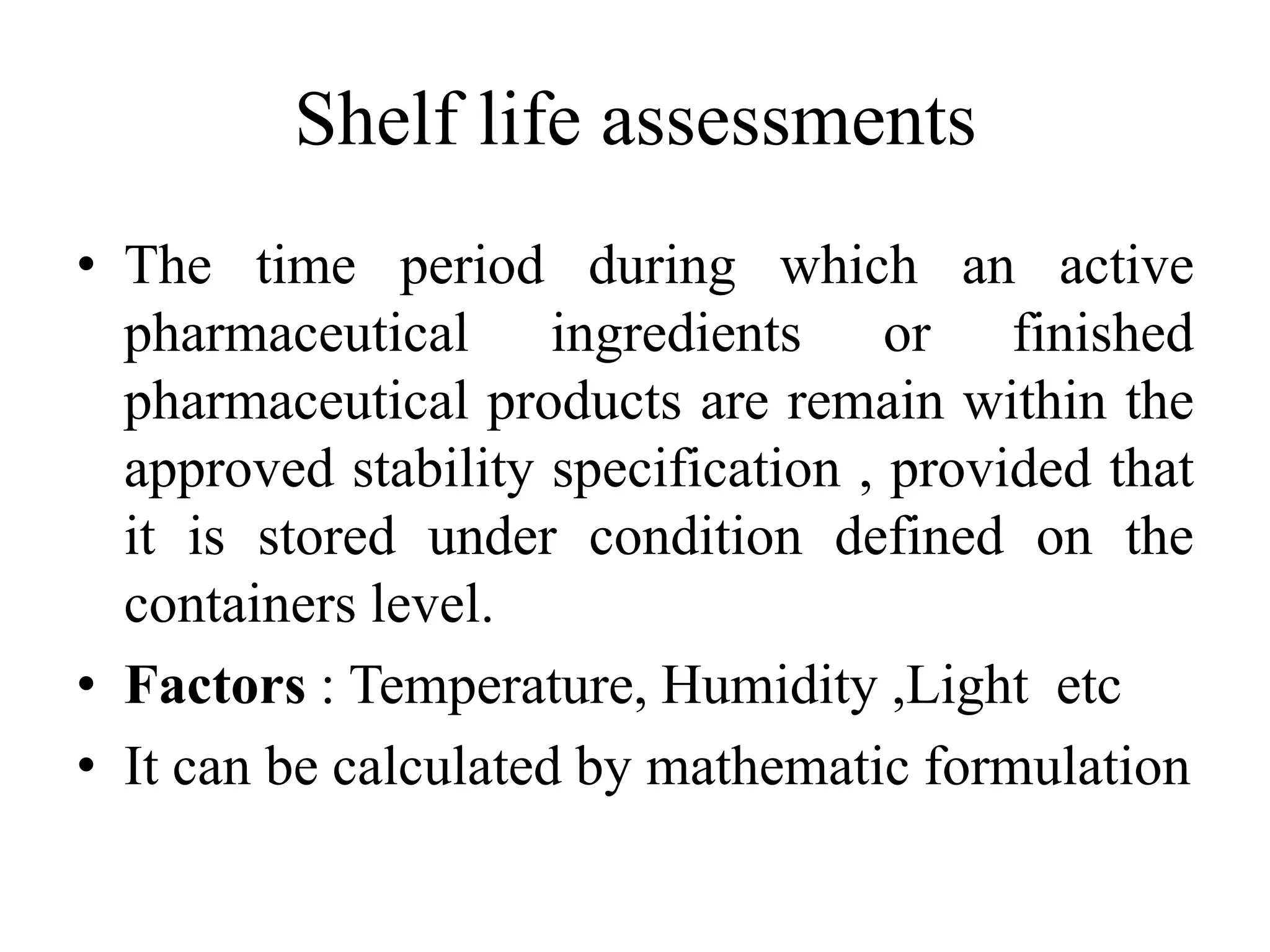
This document discusses the standardization of Ayurvedic formulations. It explains that standardization involves quantifying the purity, quality, identity and constituents of drugs and formulations. For churnas (powders), both Ayurvedic and modern parameters are used for standardization, including organoleptic evaluation, microscopy, physical analysis, phytochemical analysis, and testing for contaminants. Similar parameters are discussed for standardizing other formulations like asavas, arishtas, avalehas, vatis, gutikas, tailas, ghritas and arka. Shelf life testing is also important for ensuring formulations remain within approved specifications when stored properly.