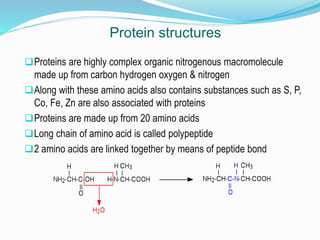
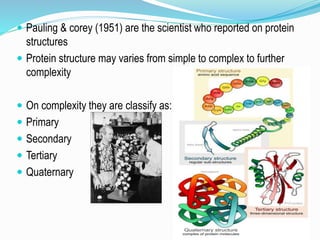
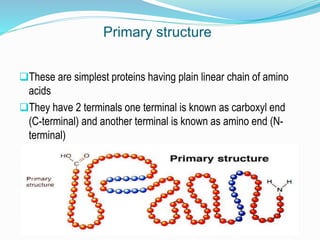
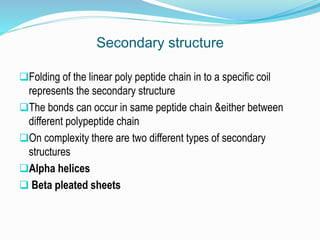
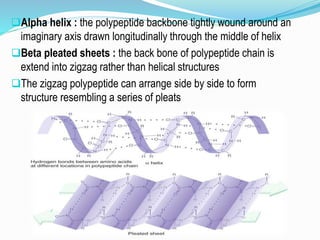
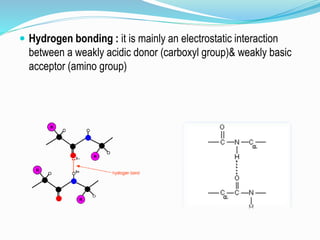
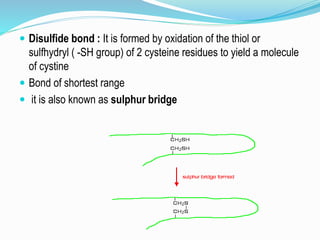
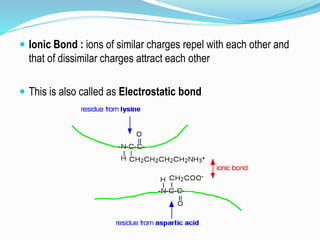
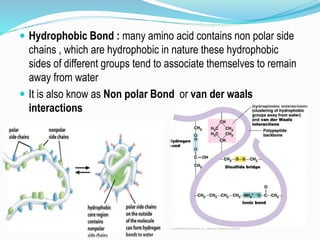
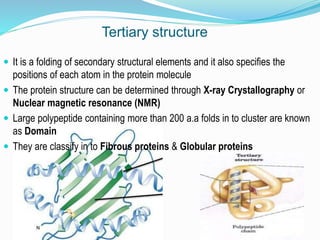
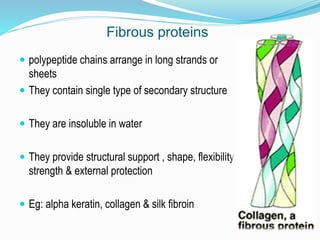
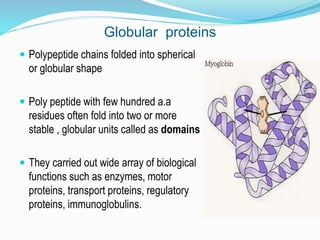
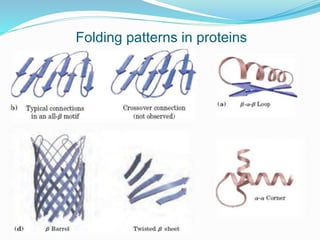
The document discusses the complex structures of proteins, which are organic macromolecules made up of amino acids and other elements. It classifies protein structures into primary, secondary, tertiary, and quaternary, detailing the characteristics and bonding of each level, such as alpha helices and beta pleated sheets. The document also differentiates between fibrous and globular proteins, explaining their structural roles and functions.