1. Scientific notation is a way to write numbers as a product of a coefficient and a power of 10 to express very large and very small numbers.
2. A law is a statement of fact based on repeated observation that describes natural phenomena, while a theory is a well-substantiated explanation of such a law.
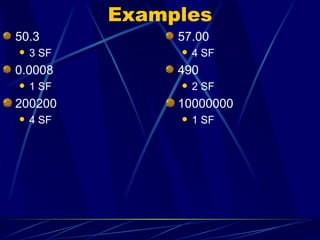
3. Significant figures indicate the precision or uncertainty of a measurement and are used to calculate the error in products and quotients of calculations.