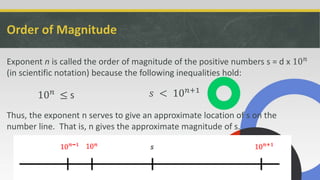
This document provides a lesson on scientific notation. It begins with examples of writing numbers like populations in scientific notation. It then gives examples of adding and subtracting numbers in scientific notation, showing how to equalize exponents. The document explains that scientific notation allows precise approximation of very large and small numbers by showing order of magnitude. It concludes that scientific notation facilitates tracking and comparing extremely large or small values.