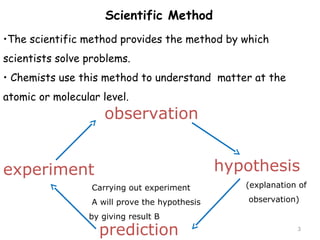
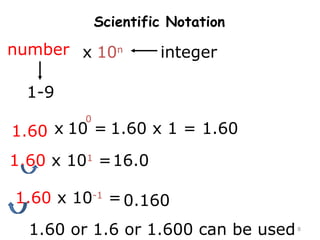
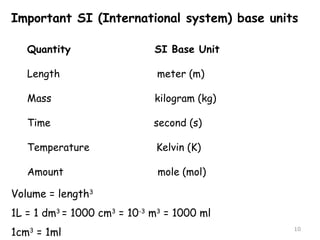
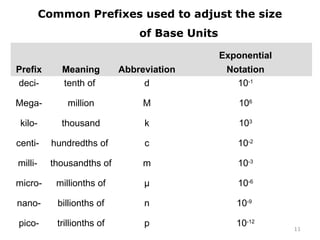
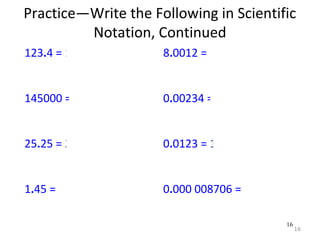
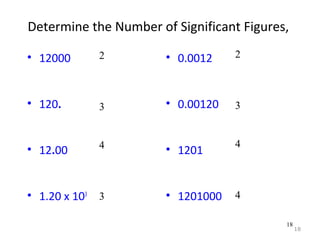
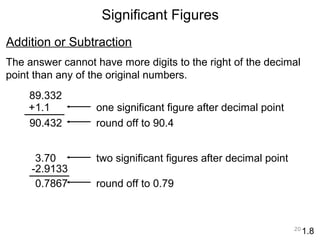
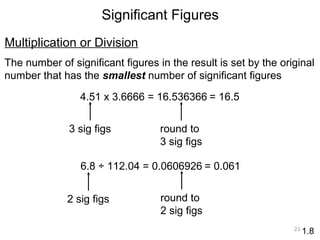
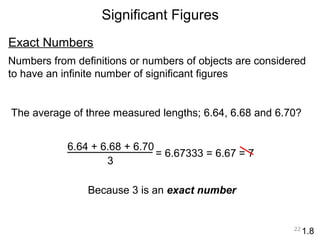
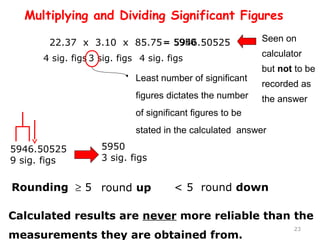
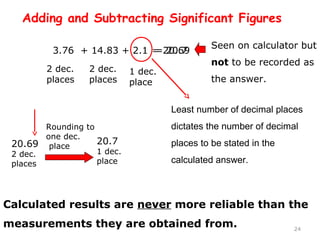
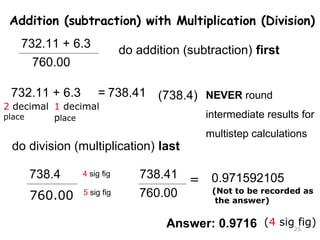
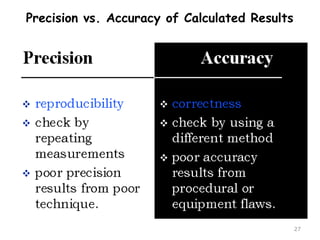
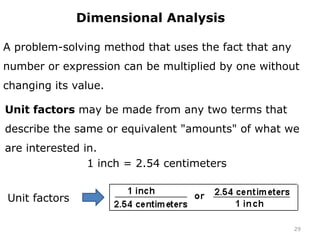
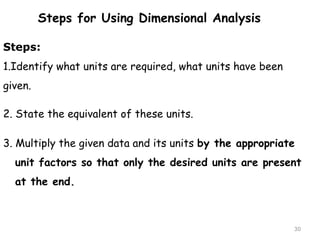
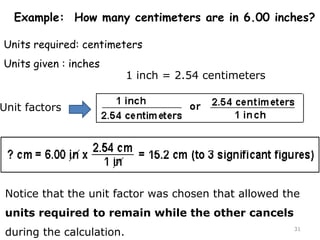
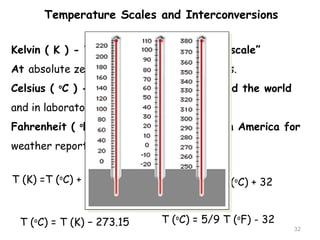
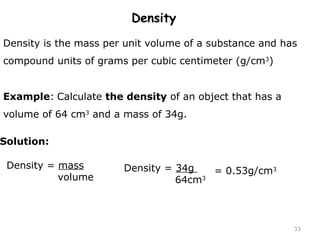
This document provides an introduction to CHEM 1101 and covers several key chemistry concepts. It introduces the instructor, Dr. Muhannad Amer, and discusses the scientific method and how it is used in chemistry. It also defines and compares scientific theories and laws. Additionally, it covers important topics such as the International System of Units (SI units), dimensional analysis, density, temperature scales, and significant figures.