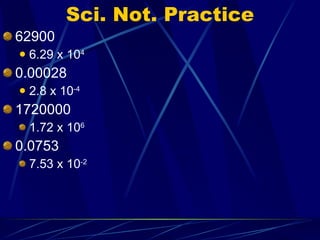
1. Scientific notation is used to express very large or very small numbers in a standard way using a coefficient and power of 10.
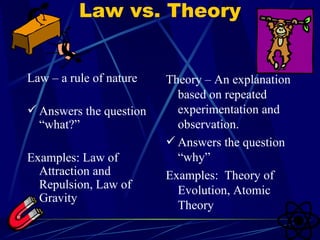
2. Theories are explanations based on repeated experimentation and observation, while laws are rules of nature. Theories do not become laws.
3. Significant figures tell us how precisely a measurement or number is known and indicate the reliability of the last digits.