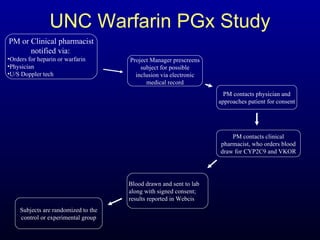
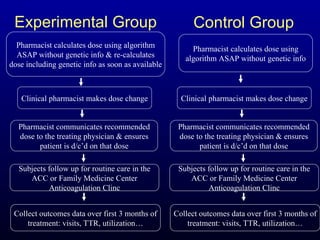
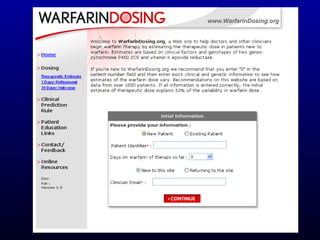
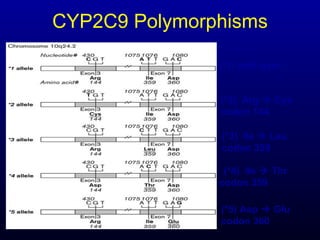
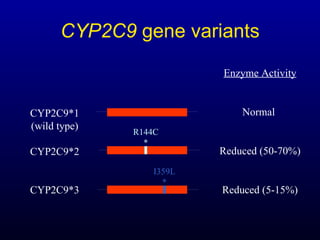
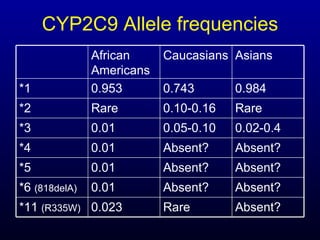
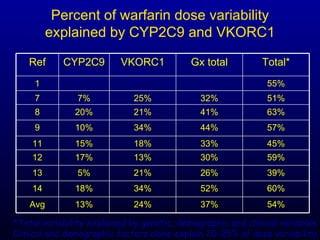
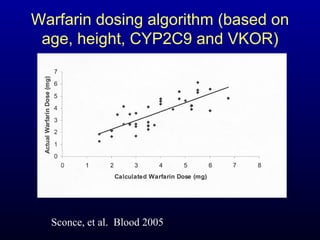
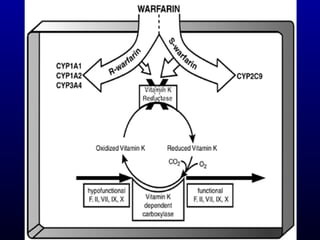
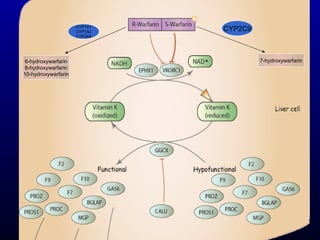
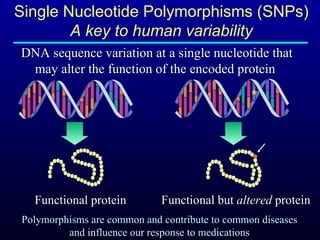
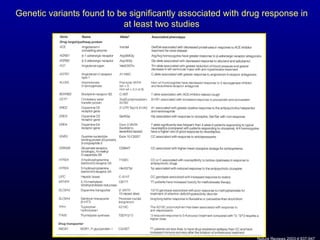
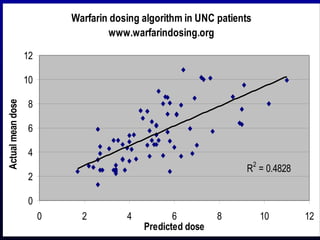
The document discusses using genetic testing to guide warfarin therapy. It explains that genetic polymorphisms affect individuals' responses to medications like warfarin. Variants in CYP2C9 and VKORC1 genes influence warfarin dosing, with clinical trials showing genotype-guided dosing results in faster stabilization of anticoagulation and less risk of bleeding events. The author proposes a study at UNC to incorporate pharmacogenomic guidance in initial warfarin dosing to improve outcomes.












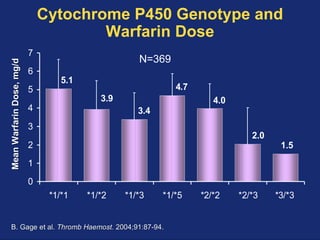





![Caraco et al. Controlled trial, prospective (“randomized” by MRN); N=283 enrolled, 191 analyzed Control group: all started on 5mg and adjust dose based on pre-established protocol Required daily monitoring of INR for initial 8 days Intervention group: CYP2C9 genotype-adjusted protocol Altered recommended dose by a set % for each of 6 different genotypes (*1/*1, *1/*2, *1/*3, etc.) Results: stable anticoagulation 18.1 days earlier TTR 80.4% vs 63.4% (P < 0.001) Clin Pharmacol Ther. 2007 Sep 12; [Epub ahead of print]; Hadassah University, Israel](https://image.slidesharecdn.com/10-29-07coumadinpgxjonas-090325164146-phpapp01/85/10-29-07-Coumadin-P-Gx-Jonas-23-320.jpg)

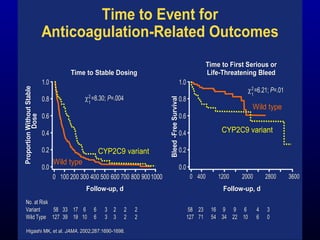
![Limdi et al. Large prospective cohort study (N=490) with 2 year follow up All patients treated with standardized approach to warfarin dose adjustments Results: Variant CYP2C9 genotype Increased risk for major hemorrhage (HR 3.0; 95% CI 1.1-8.0) a , but not minor hemorrhage Variant VKORC1 genotype (1173C/T) Did not confer an increase in risk for major or minor Limidi et al., Clin Pharmacol Ther. 2007 Jul 25; [Epub ahead of print]; UAB a Adjusted for age, gender, race, BMI, VKORC1, vitamin K and alcohol intake, warfarin dose, interacting drugs, number of comorbid conditions, and INR at the time of the event](https://image.slidesharecdn.com/10-29-07coumadinpgxjonas-090325164146-phpapp01/85/10-29-07-Coumadin-P-Gx-Jonas-25-320.jpg)