- Ovarian cancer is the 4th leading cause of cancer death in women in the US, with a 5-year survival rate of only 35% for advanced cases. Most cases are diagnosed at an advanced stage due to non-specific early symptoms.
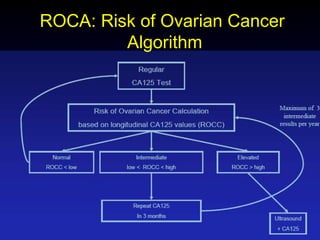
- There is no consensus on screening guidelines due to a lack of evidence that screening reduces mortality. Current screening methods like ultrasound and CA-125 lack sensitivity and specificity.
- Several large trials are underway to evaluate new screening strategies using ultrasound, tumor markers, and genetic testing to enable earlier detection when treatment is most effective. Improved screening methods are needed to reduce ovarian cancer mortality rates.