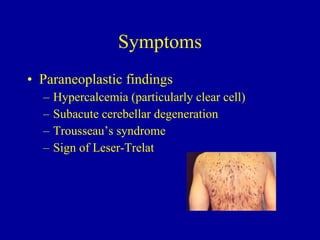
Ovarian cancer is the 8th most common cancer in women and the 5th leading cause of cancer death. It has a median age of diagnosis of 60 years old and 68% of cases are metastatic at diagnosis. Risk factors include family history, personal history of breast cancer, infertility, and lack of pregnancy. Genetic mutations like BRCA1/2 account for 10-15% of cases. Symptoms are often vague. Treatment involves surgical staging and debulking followed by platinum-based chemotherapy, with the goal of optimal cytoreduction to ≤1cm residual disease.