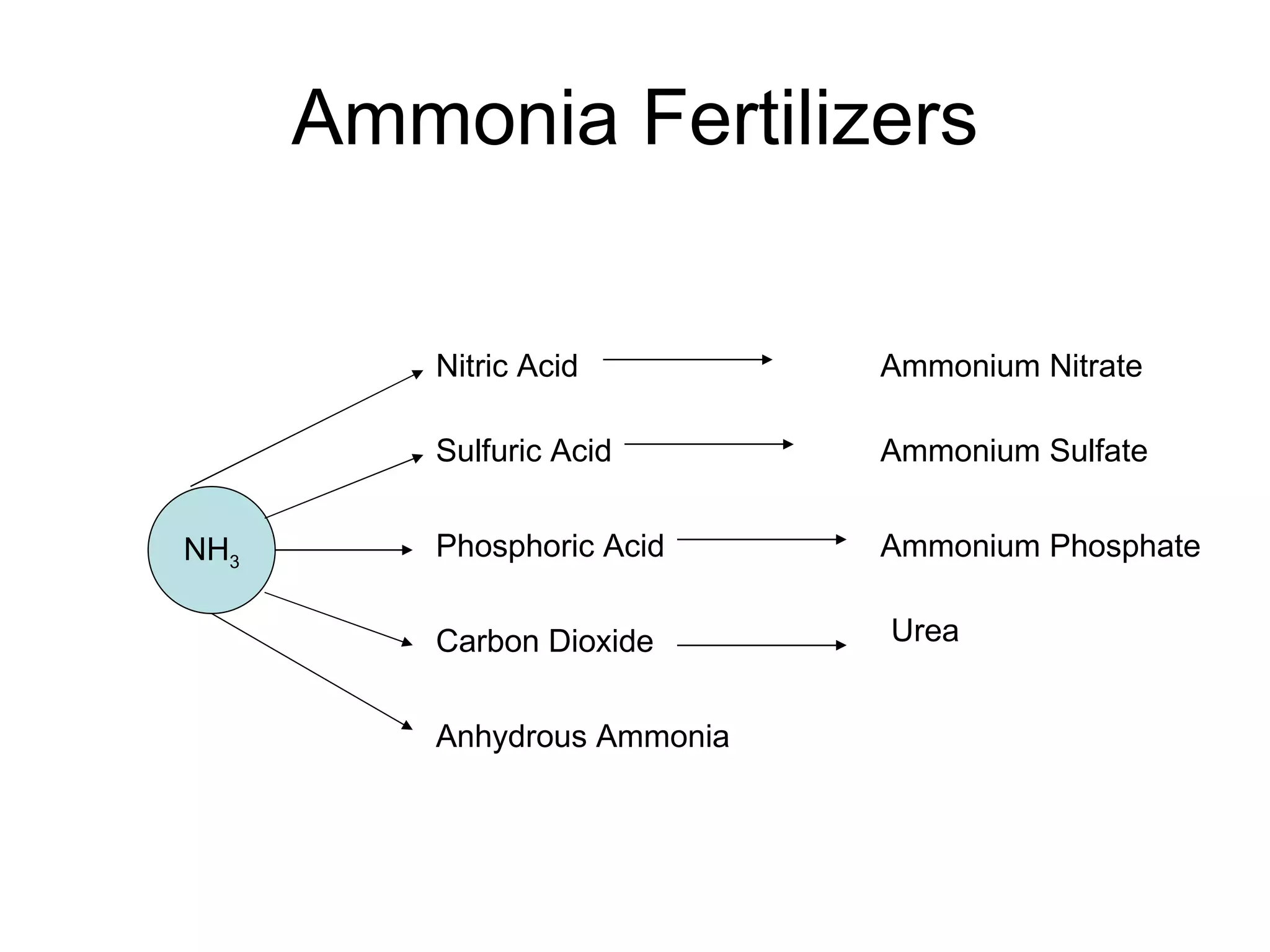
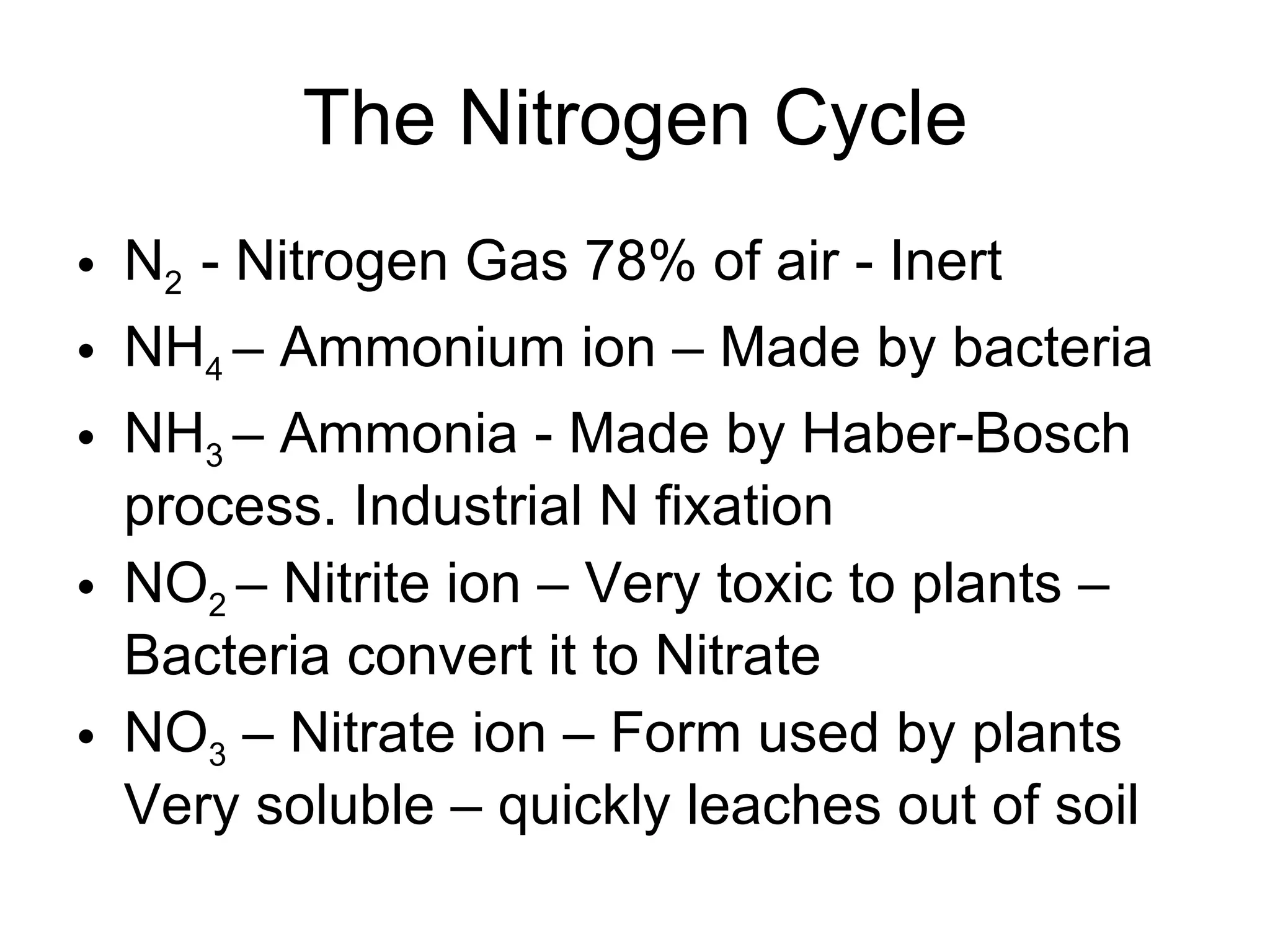
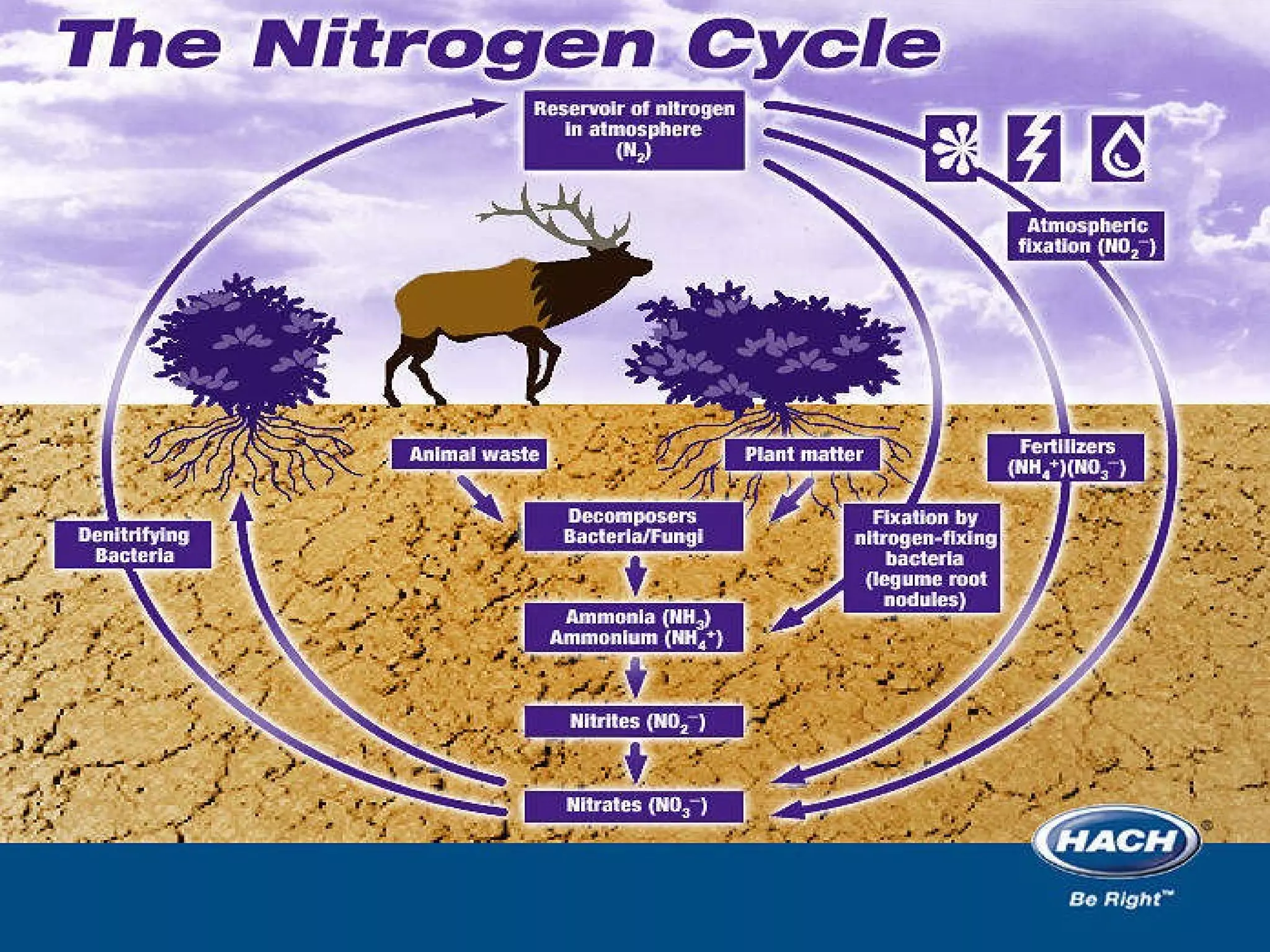
This document provides an overview of the nitrogen cycle and legumes. It discusses how legumes have a symbiotic relationship with rhizobia bacteria in their roots that fix nitrogen. It also explains the processes of nitrogen fixation, nitrification, denitrification, volatilization, decay, and the carbon-nitrogen ratio in relation to the nitrogen cycle. Key points covered include how rhizobia are specific to different legume species and that naturalized rhizobia can be as effective as commercial inoculants in fixing nitrogen.