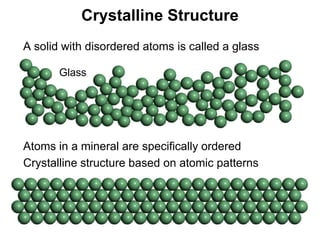
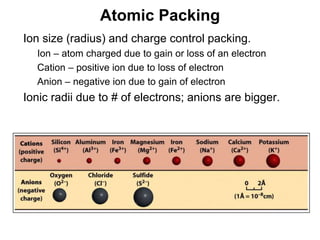
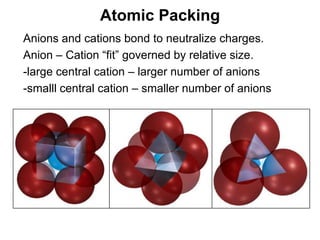
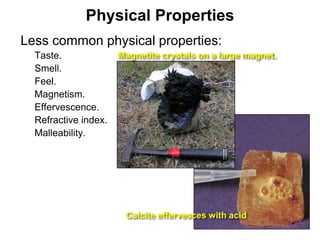
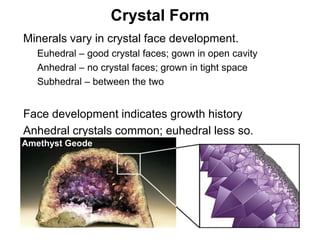
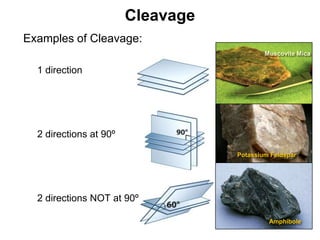
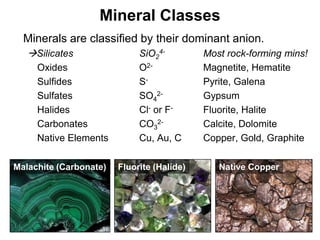
The document discusses minerals and rocks. It defines minerals as naturally occurring solids with a crystalline structure and specific chemical composition, while rocks are aggregates of minerals. There are over 4000 known minerals that are divided into classes based on their dominant chemical component, most importantly the silicates. Silicates are made of silica tetrahedra that bond together in different ways to form distinct mineral groups with varying properties. The document outlines several important physical properties used to identify minerals such as color, crystal structure, hardness, and cleavage.