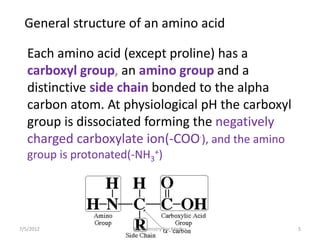
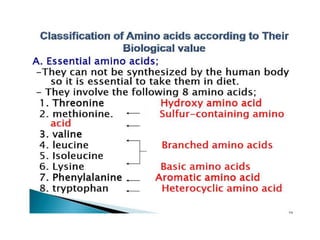
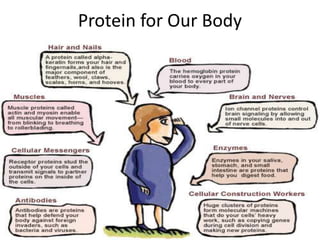
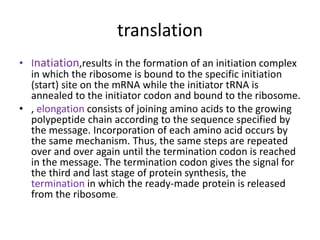
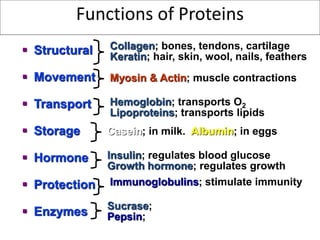
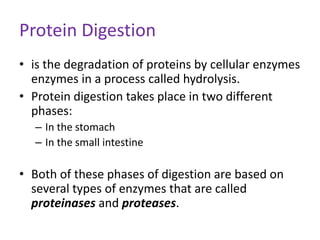
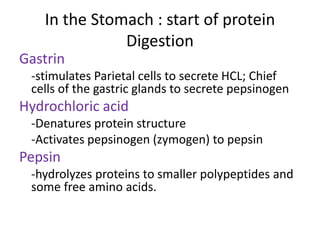
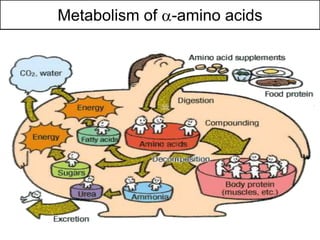
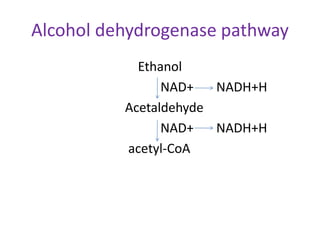
Proteins are composed of amino acids and perform many essential functions in living organisms. There are 20 standard amino acids that make up proteins. Proteins are broken down into amino acids through digestion in the stomach and small intestine by enzymes like pepsin and trypsin. Amino acids are then absorbed into the bloodstream and transported to tissues. Insufficient protein intake can lead to protein-energy malnutrition conditions like marasmus and kwashiorkor. Alcohol is also broken down through metabolic pathways in the liver involving enzymes. Excessive alcohol consumption can cause serious health issues like liver disease and cancer.