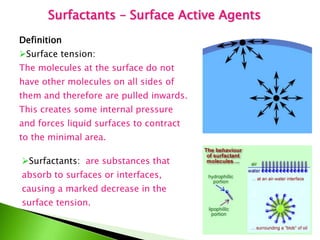
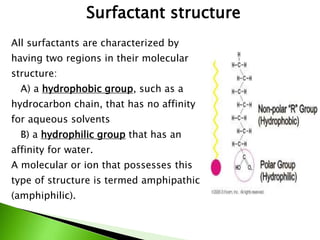
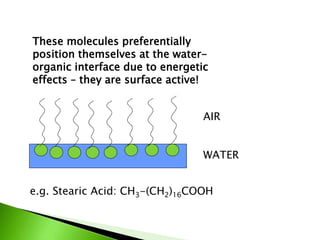
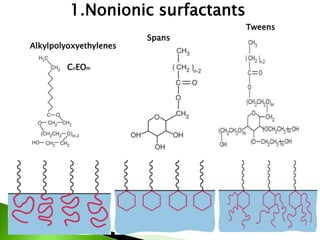
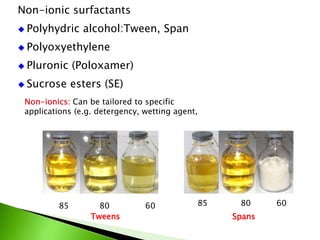
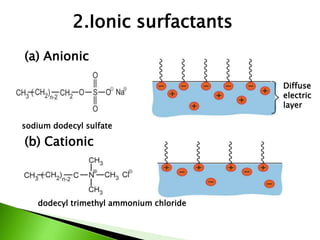
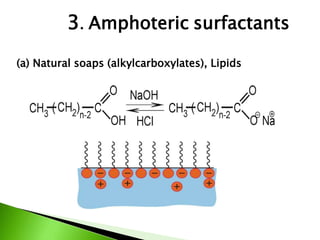
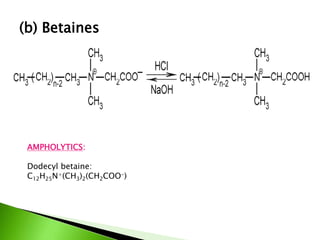
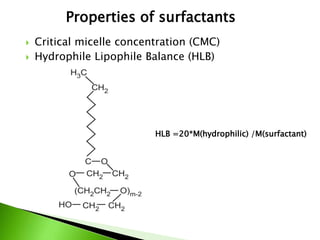
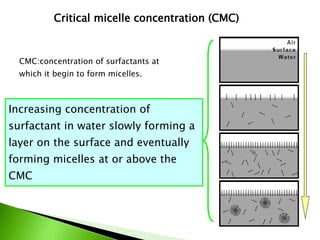
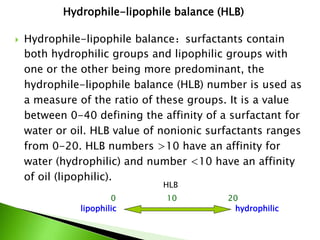
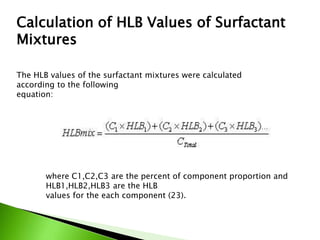
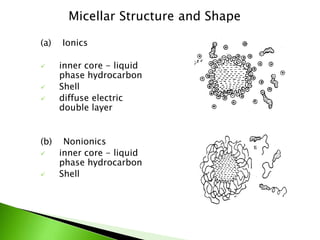
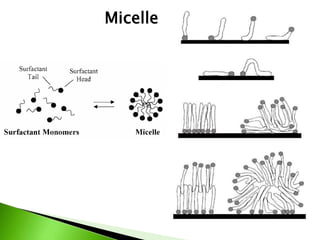
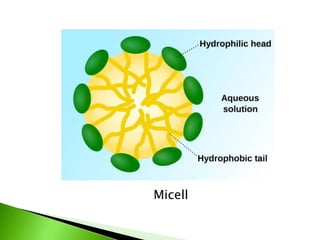
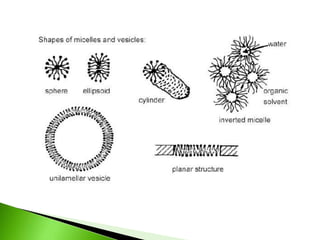
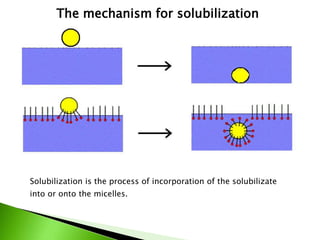
The document defines surfactants as substances that decrease surface tension by absorbing to surfaces/interfaces. Surfactants have a hydrophilic group that attracts water and a hydrophobic group that repels water. This amphipathic structure allows surfactants to position at water-organic interfaces. Surfactants are classified as ionic (anionic, cationic), nonionic, or amphoteric depending on their head groups. Key properties include critical micelle concentration (CMC) and hydrophile-lipophile balance (HLB). Surfactants have many applications including detergents, personal care products, paints, food processing, and mining.