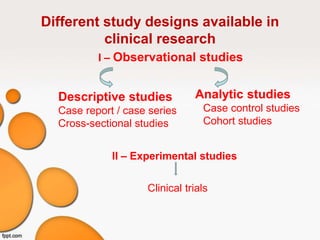
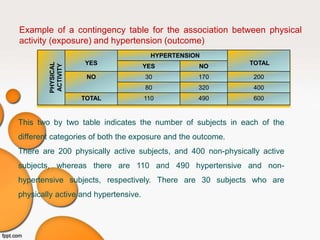
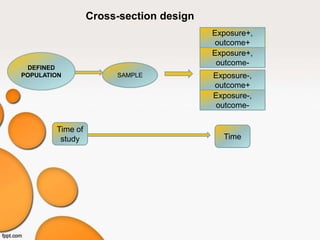
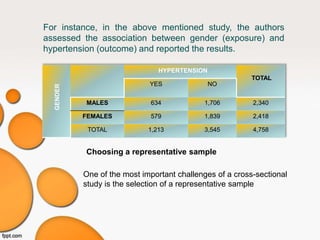
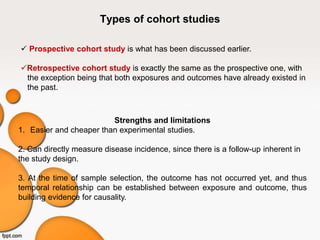
This document provides an overview of different study designs used in clinical research. It describes descriptive studies like case reports, case series, and cross-sectional studies which aim to describe characteristics of populations. Analytical studies like case-control and cohort studies assess associations between exposures and outcomes. Experimental studies like randomized clinical trials allocate exposures to subjects. Biases like selection bias, information bias, and confounding are discussed. An example of a cross-sectional study design assessing hypertension prevalence is provided.