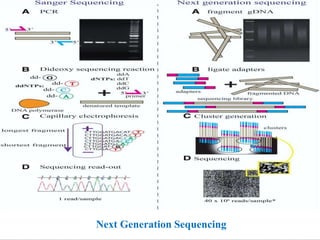
The document outlines the structure and mutation of the human genome, which contains approximately 20,000 to 25,000 genes across 23 chromosome pairs. It discusses various genetic disorders classified by their genetic basis, including mutations, and emphasizes the role of diagnostic techniques such as PCR and DNA sequencing in identifying mutations for effective treatment. Additionally, it details methods for mutation detection and prediagnostic approaches, underscoring the importance of accurate genetic diagnosis for disease management.