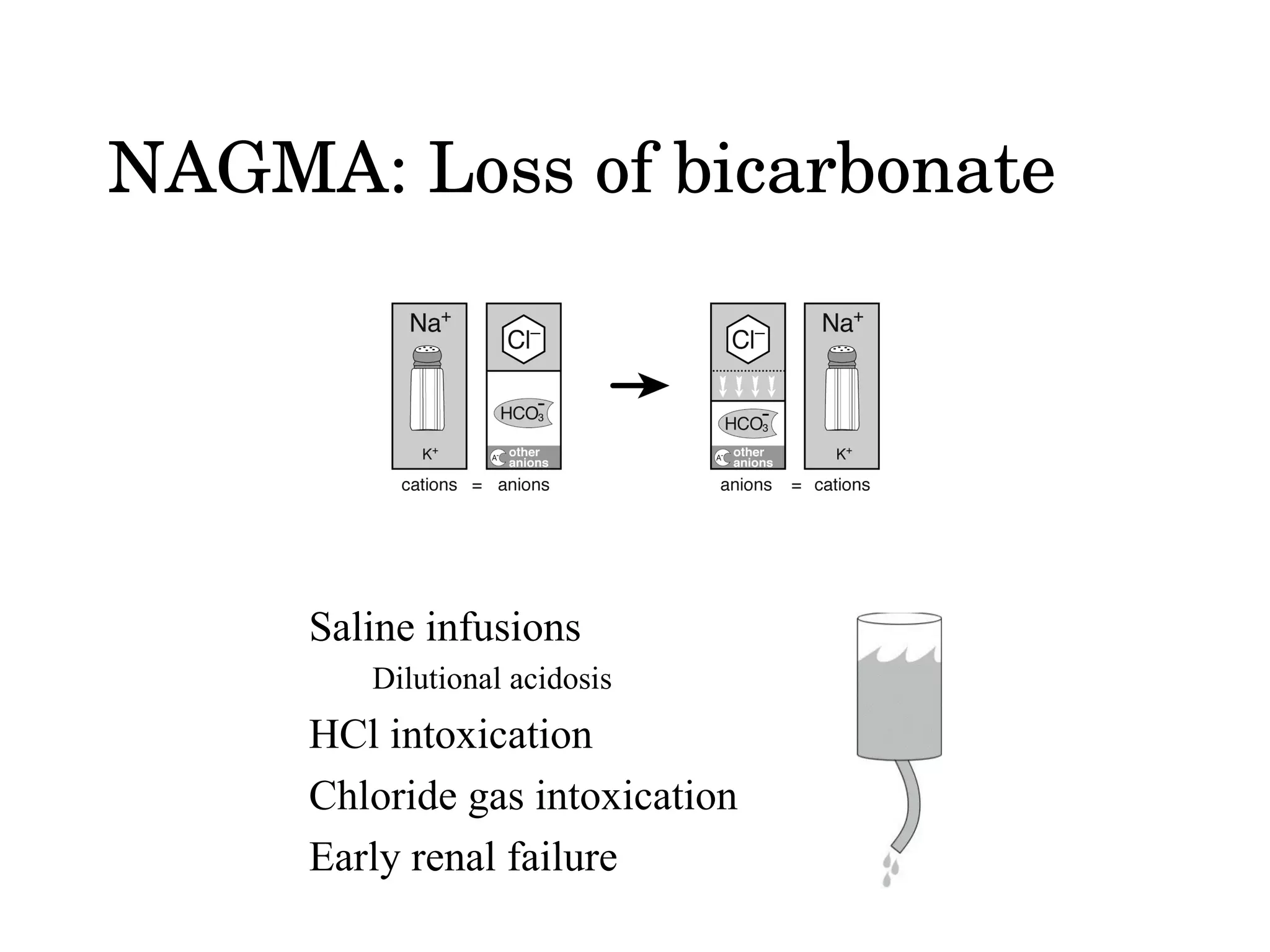
The document discusses different types of metabolic acidosis, including non-anion gap metabolic acidosis (NAGMA) and renal tubular acidosis (RTA). It provides details on evaluating acid-base imbalances and determining if a metabolic acidosis is respiratory or metabolic. Causes of NAGMA include loss of bicarbonate from the GI tract or kidneys. Proximal and distal RTA can result from different defects in renal bicarbonate reabsorption and new bicarbonate production.