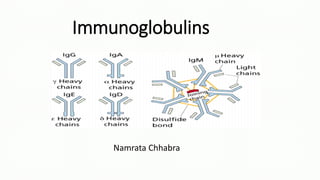
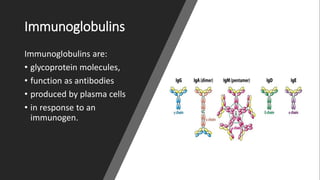
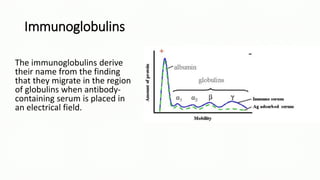
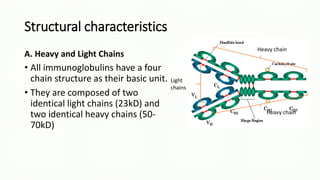
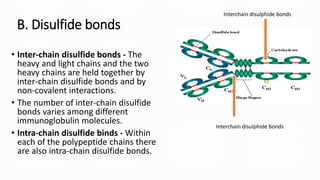
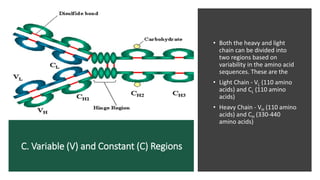
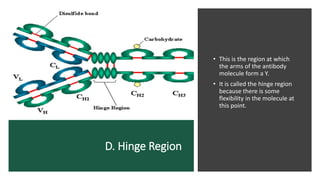
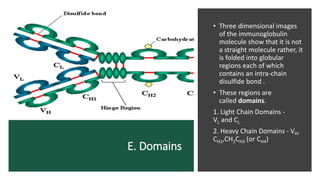
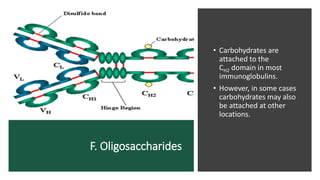
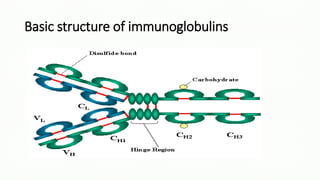
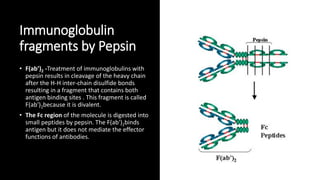
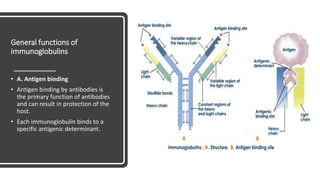
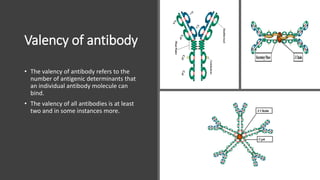
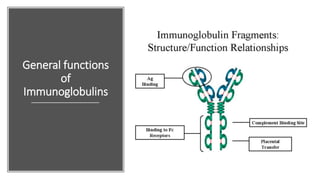
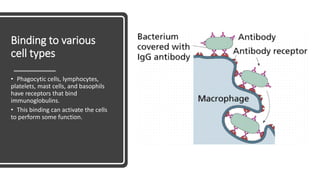
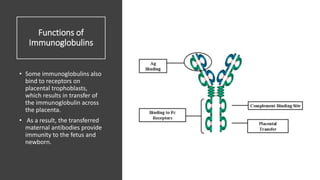
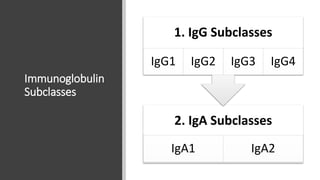
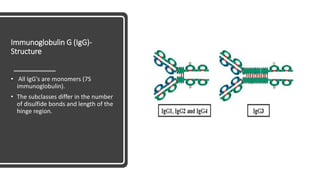
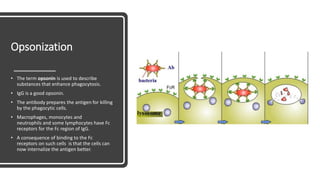
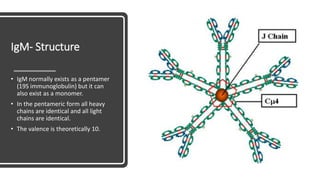
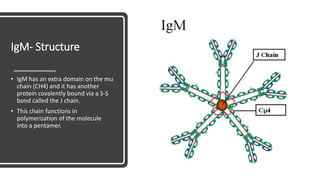
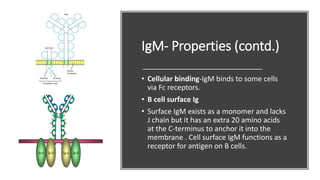
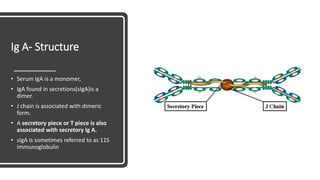
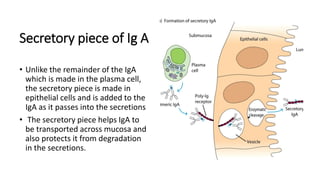
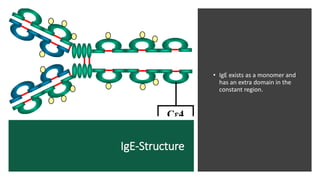
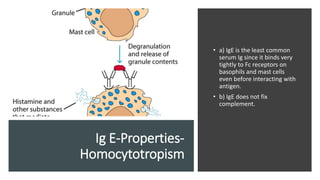
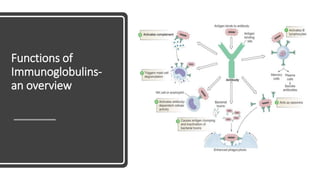
Immunoglobulins, also known as antibodies, are glycoprotein molecules produced by plasma cells that function to bind to antigens. There are five main classes of immunoglobulins - IgG, IgM, IgA, IgD, and IgE - which differ in their structure and functions. IgG is the most abundant immunoglobulin found in serum and tissues. It can activate complement and promote opsonization and phagocytosis. IgM is the first immunoglobulin produced during a primary infection and helps activate the complement system. IgA is important for mucosal immunity as the main immunoglobulin found in secretions like tears and saliva.