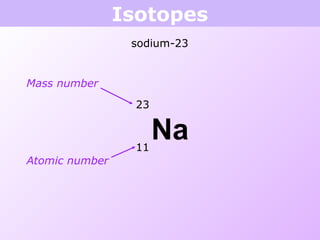
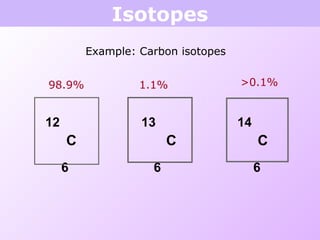
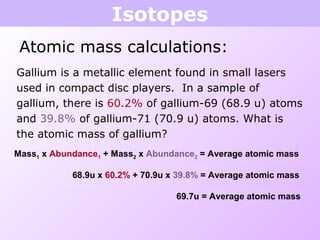
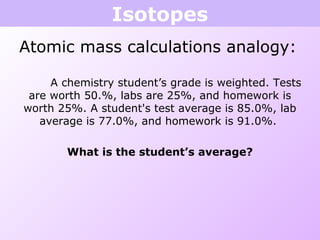
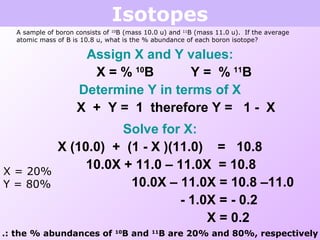
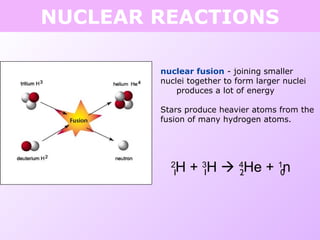
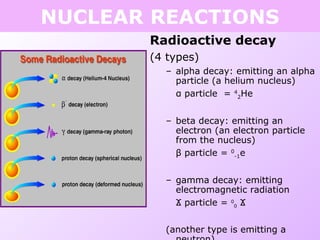
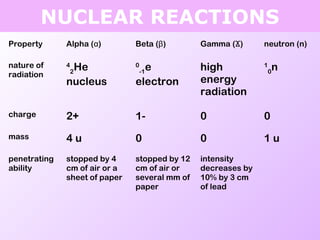
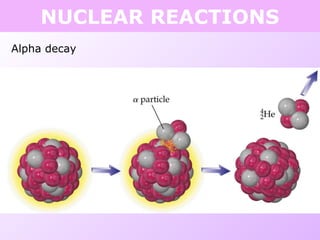
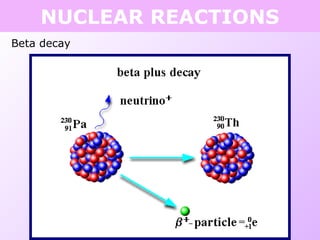
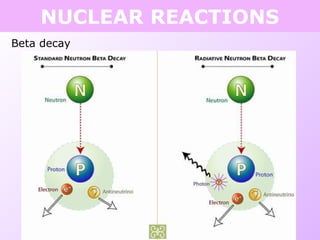
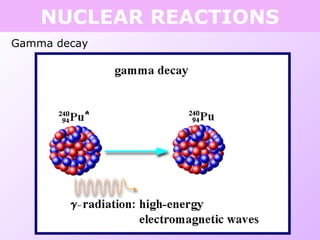
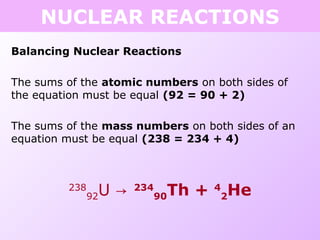
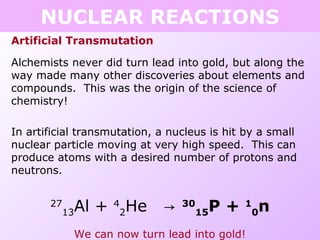
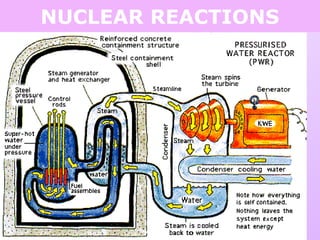
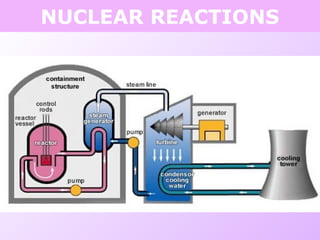
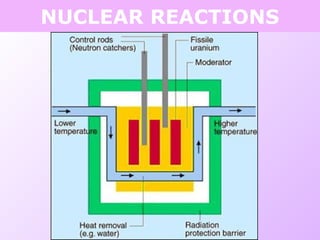
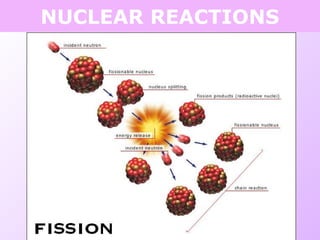
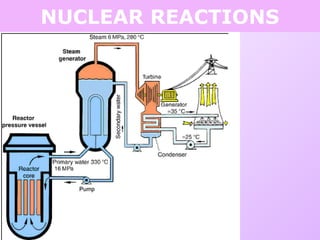
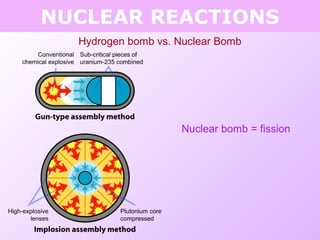
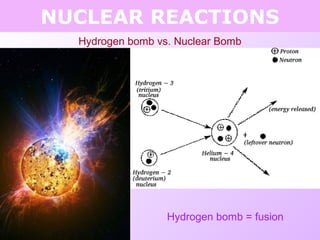
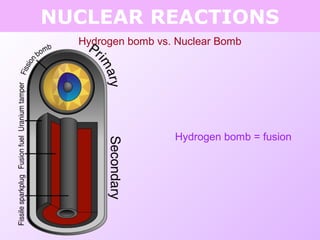
This document discusses isotopes and nuclear reactions. It defines isotopes as atoms of the same element with different numbers of neutrons. It also describes how atomic mass is calculated based on isotope abundances. The document then discusses four types of nuclear reactions: fusion, fission, alpha decay, and beta decay. It provides examples of writing balanced nuclear equations and calculating half-life. Artificial transmutation and uses of nuclear technology like reactors and weapons are also summarized.