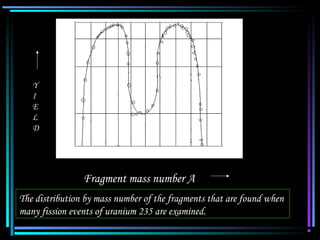
This document discusses nuclear reactions and different types of nuclear processes including nuclear fission and nuclear fusion. It explains that nuclear reactions occur when atomic nuclei interact, changing the composition but conserving mass and charge. Nuclear fission is the splitting of heavy atomic nuclei after absorbing neutrons, releasing energy, while nuclear fusion is the combining of light nuclei into heavier ones. Controlled nuclear fusion research aims to replicate the energy process of the sun for energy production on Earth.