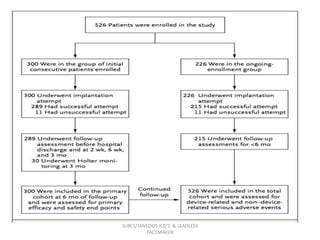
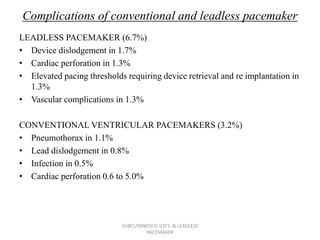
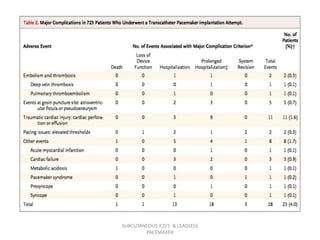
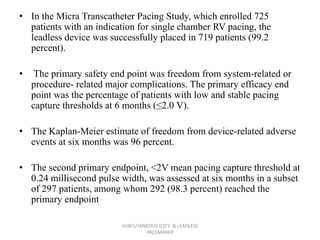
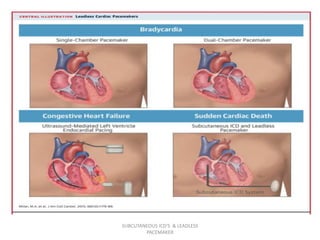
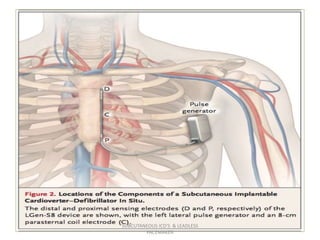
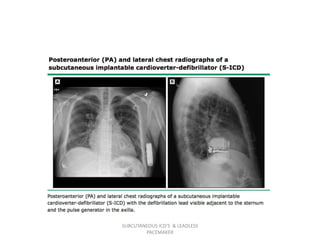
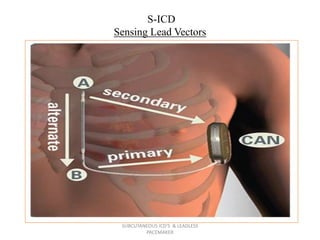
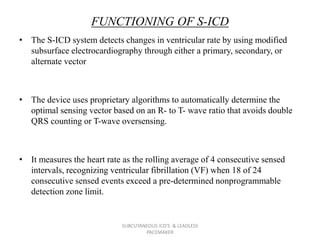
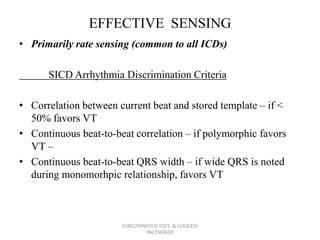
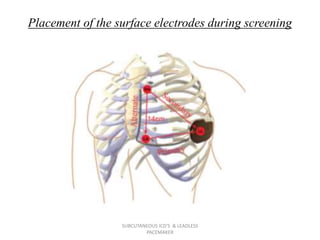
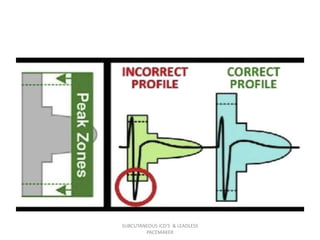
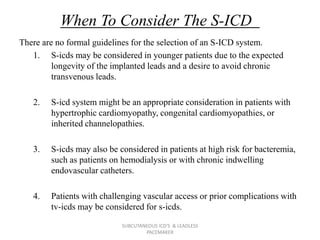
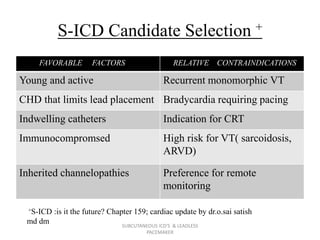
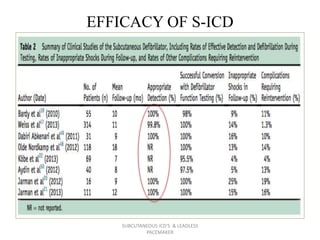
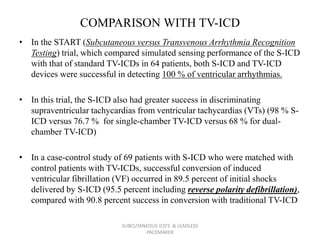
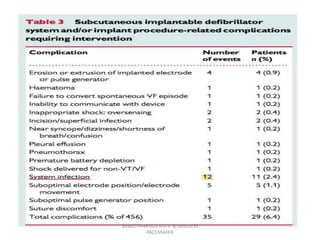
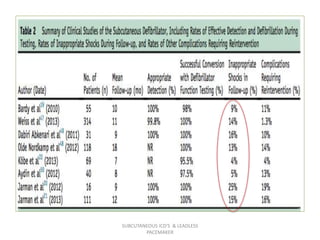
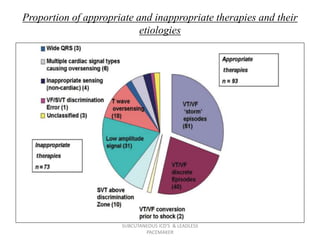
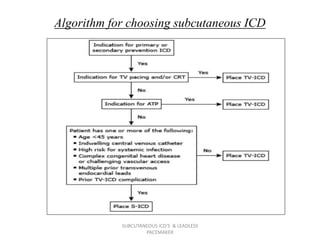
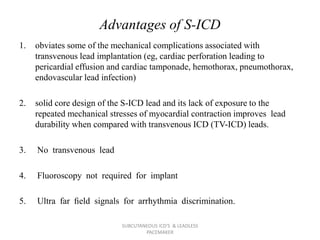
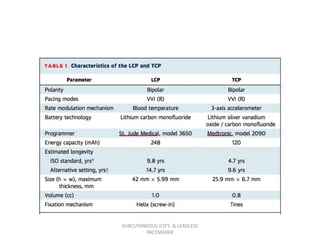
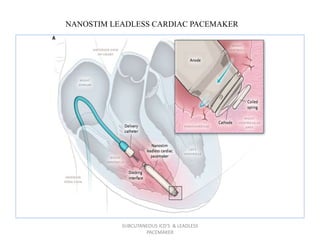
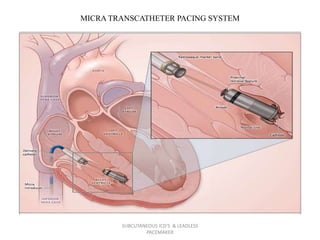
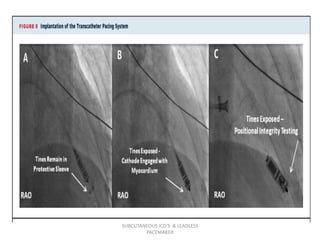
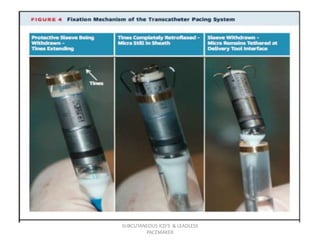
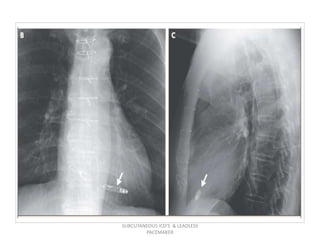
The document discusses subcutaneous implantable cardioverter defibrillators (S-ICDs) and leadless pacemakers as alternatives to transvenous ICD systems. S-ICDs avoid the risks of transvenous leads but do not provide antitachycardia pacing or bradycardia support. Studies show S-ICDs effectively detect and treat ventricular arrhythmias similar to transvenous ICDs. However, S-ICDs have a higher risk of inappropriate shocks and pocket infections compared to transvenous ICDs. Leadless pacemakers eliminate transvenous leads but have not yet demonstrated long-term reliability.


































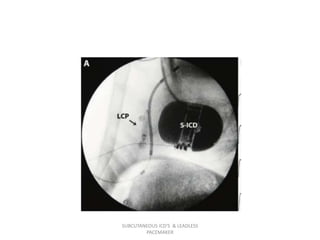
![LEADLESS Trial
• The, a first-in-human, single-arm, multicenter study of the safety and
clinical performance of the LCP.
• patients were considered eligible if they had indications for single-chamber,
right ventricular pacing (VVI [R]).
Indications included:
1) permanent atrial fibrillation with atrioventricular block (including atrial
fibrillation with a slow ventricular response).
2) normal sinus rhythm with second- or third-degree atrioventricular block
with a low level of physical activity or short expected life span.
3) sinus bradycardia with infrequent pauses or un- explained syncope with
electrophysiologic findings.
SUBCUTANEOUS ICD'S & LEADLESS
PACEMAKER](https://image.slidesharecdn.com/seminarpacemaker-180722063813/85/LEADLESS-PACEMAKER-AND-SUBCUTANEOUS-ICD-60-320.jpg)