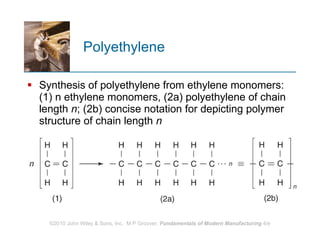
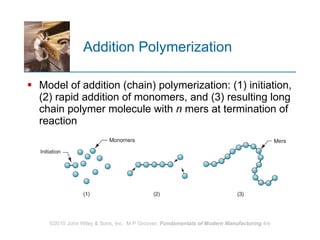
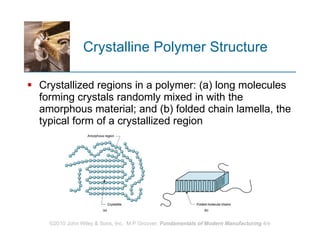
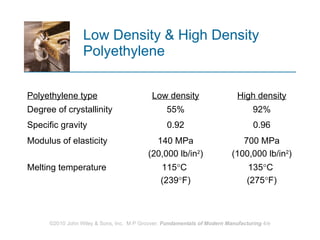
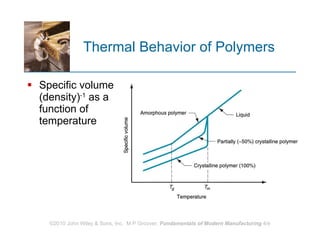
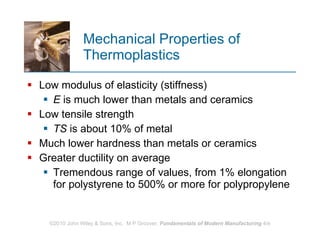
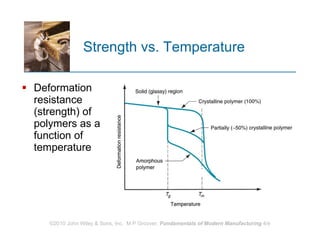
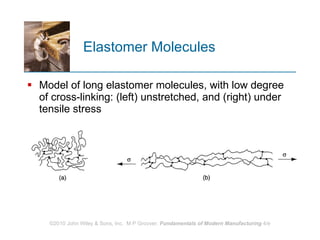
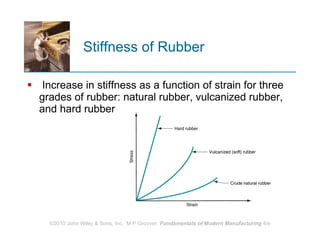
The document discusses different types of polymers including thermoplastics, thermosets, and elastomers. It provides details on their molecular structures, properties, production methods, and common applications. Thermoplastics are solid but soften when heated, thermosets permanently harden when heated, and elastomers can stretch greatly and recover their original shape. Additives are used to modify polymer properties for different applications. The largest markets are thermoplastics like polyethylene and polypropylene for products and packaging.