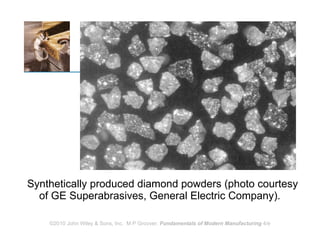
The document discusses various topics related to ceramics:
1) It defines ceramics as inorganic compounds consisting of metals and nonmetals, and provides examples such as silica and alumina.
2) It describes the properties of ceramic materials like hardness, strength at high temperatures, and brittleness.
3) It lists common ceramic products including pottery, bricks, glass, abrasives, and electrical insulators.