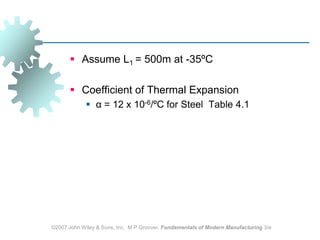
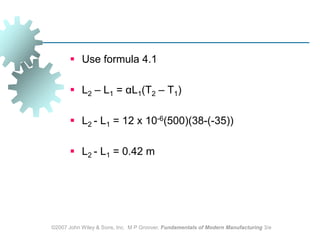
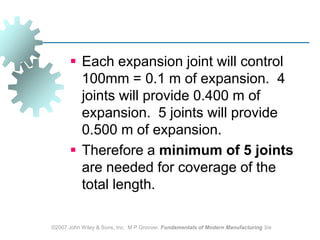
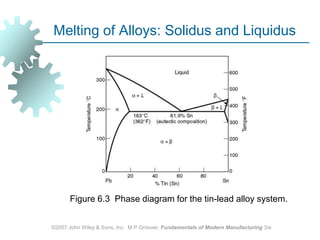
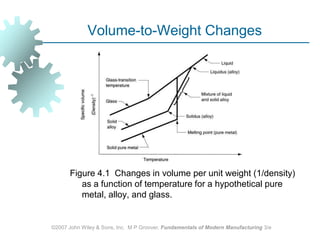
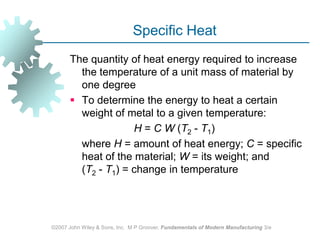
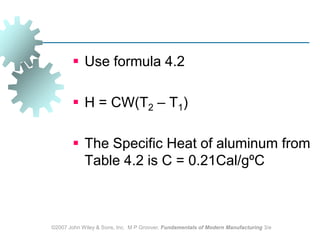
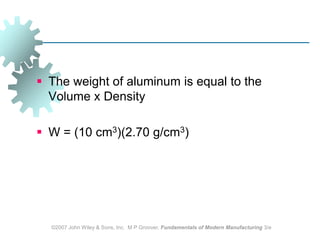
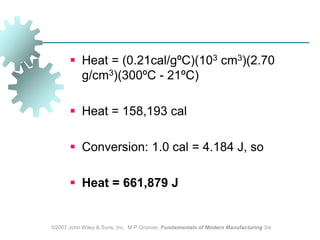
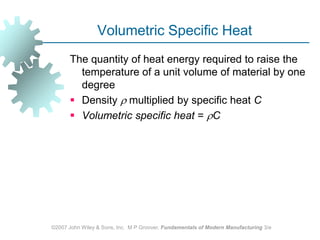
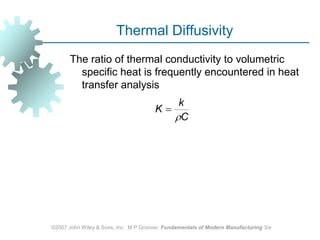
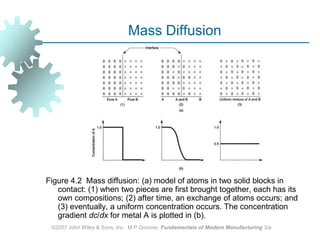
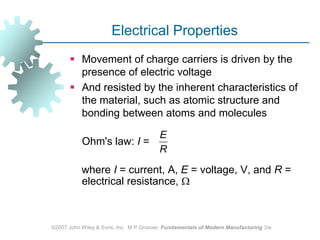
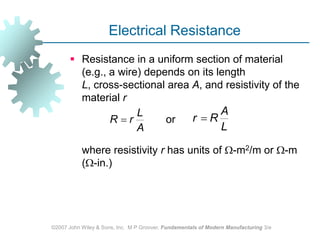
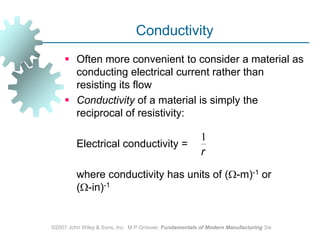
This document discusses various physical properties of materials important for manufacturing processes. It covers volumetric properties like density and thermal expansion, thermal properties including specific heat and thermal conductivity, and how these influence manufacturing. Density, thermal expansion, and melting points determine suitability for casting and other high-temperature processes. Specific heat and thermal conductivity impact the flow of heat within materials during operations like machining. Understanding these physical properties allows selection of appropriate materials for different manufacturing applications.