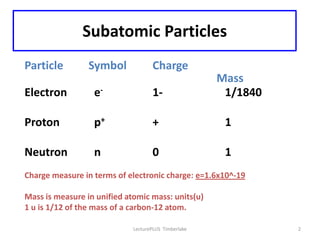
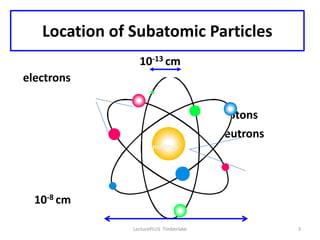
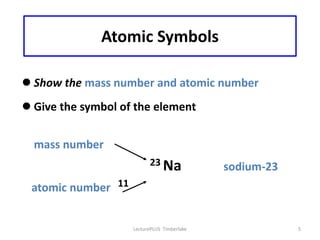
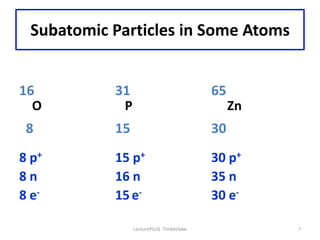
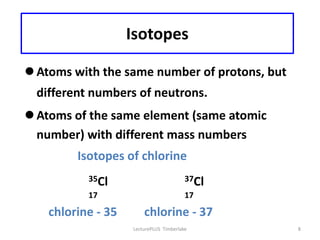
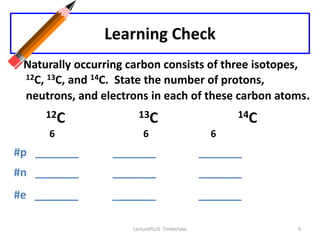
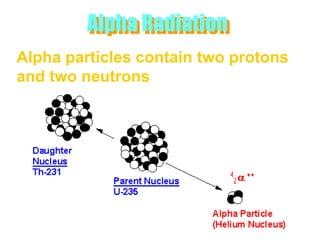
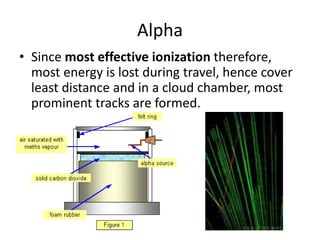
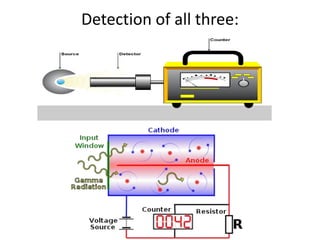
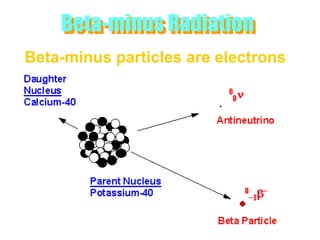
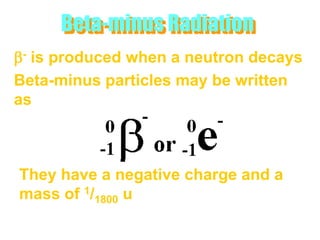
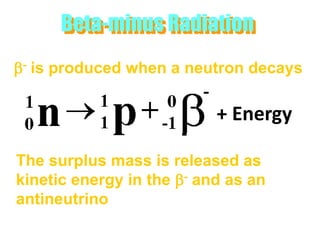
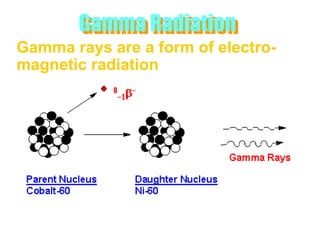
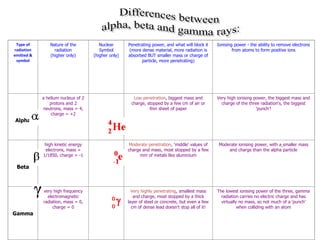
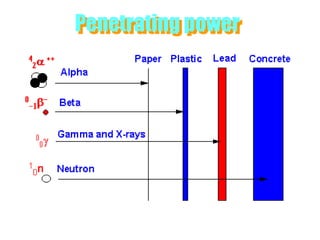
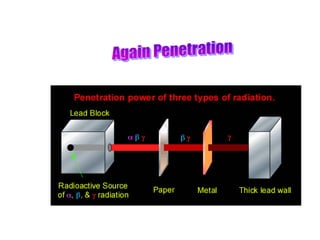
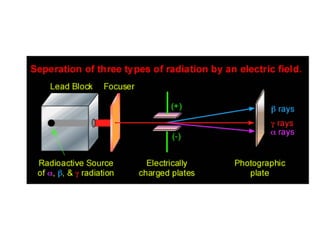
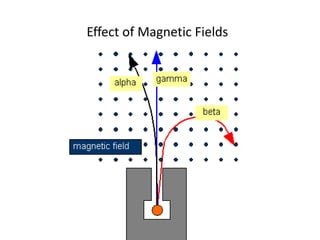
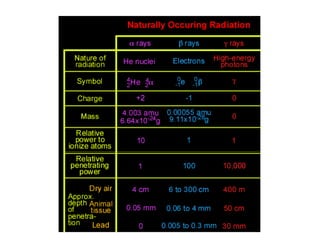
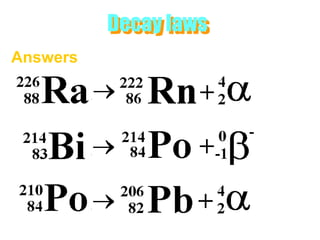
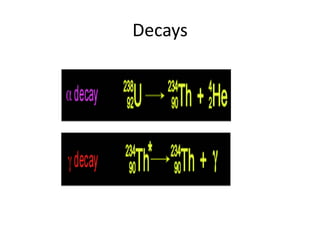
Atoms are made up of protons, neutrons, and electrons, with the number of protons defining the element. Different isotopes of an element have the same number of protons but different numbers of neutrons. Radioactive decay occurs spontaneously as nuclei emit alpha, beta, or gamma radiation to become more stable, conserving nuclear particles but decreasing mass.