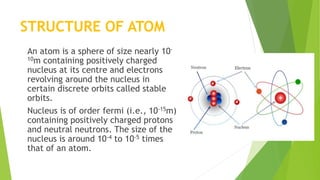
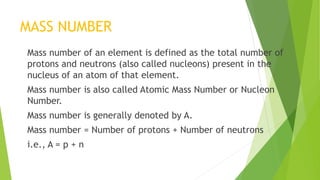
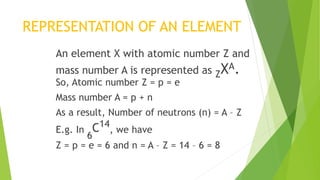
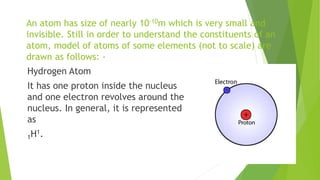
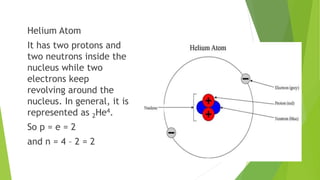
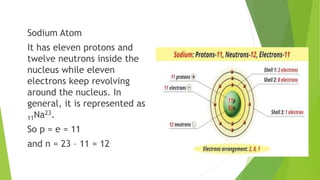
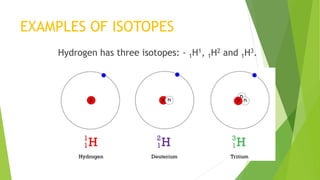
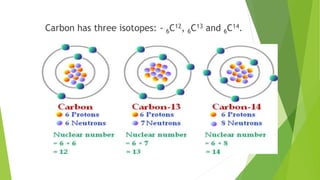
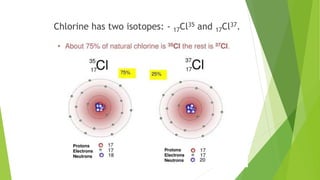
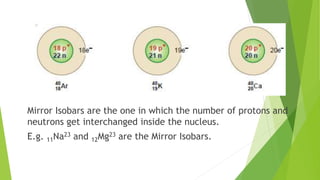
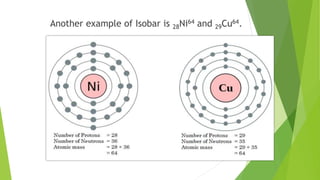
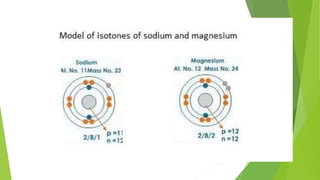
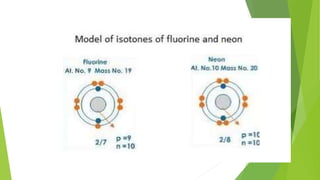
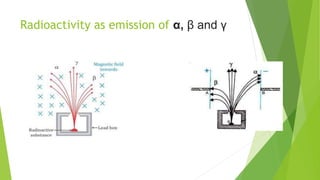
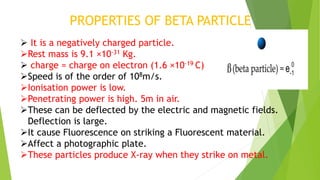
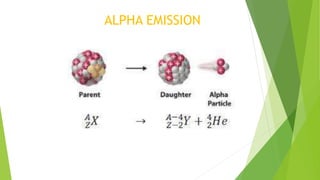
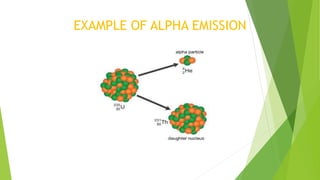
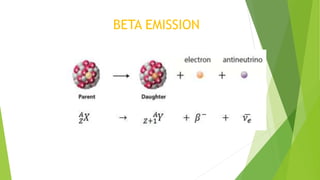
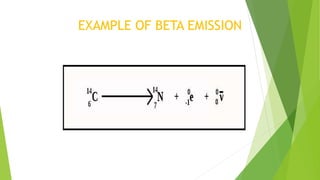
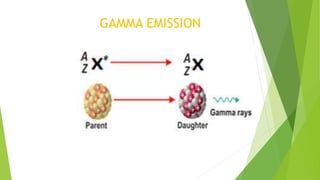
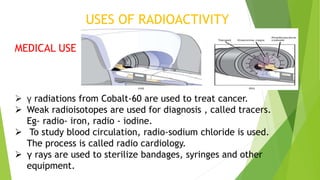
This document provides information about radioactivity and atomic structure. It begins by defining the basic structure of an atom, including the nucleus, protons, neutrons, and electrons. It then discusses atomic number, mass number, isotopes, isobars, and isotones. The document outlines the three types of radioactive emissions - alpha, beta, and gamma - and provides examples. It discusses radioactive decay and isotopes. Finally, it covers applications of radioactivity in medicine, science, and industry, as well as harmful effects of radiation and safety precautions.