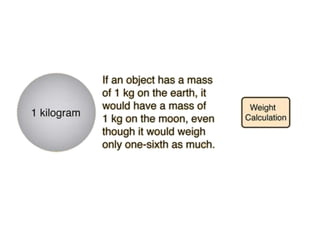
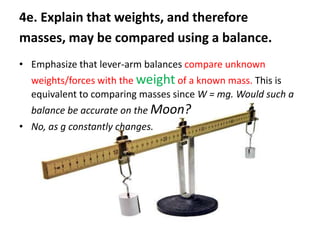
1) Mass is a measure of the amount of matter in an object and remains constant, while weight is the force exerted on an object by gravity and can vary depending on location.
2) Inertia is an object's resistance to changes in motion, and is directly proportional to its mass. Heavier objects are harder to speed up or slow down.
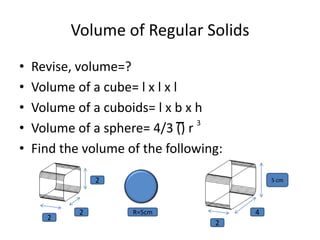
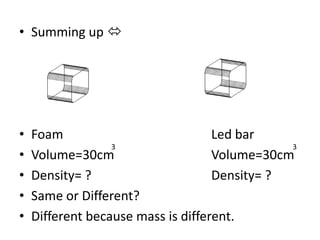
3) Density is a measure of how concentrated mass is in a substance or object, calculated as mass divided by volume. It can be used to determine if objects will float or sink in liquids. Various tools like balances and measuring cylinders are used to determine mass, volume, and density of objects.